A study reports evidence of antitumour activity by combining a GDF-15 neutralising antibody with PD-1 inhibition in cancers that are refractory/relapsed to immune checkpoint inhibitors
Encouraging results from the first-in-human GDFATHER-1/2a trial of visugromab in combination with nivolumab were presented at the ESMO Immuno-Oncology Congress 2023 (Geneva, 6–8 December) (Abstract 118O).
Patients with advanced stage/late-line cancer were included with strict criteria related to previous immunotherapy: they were either primary refractory or relapsed on continued checkpoint inhibitor therapy after an initial response, and all patients had 12 weeks’ minimum continuous anti-PD-1/-PD-L1 exposure before enrolment. Out of 174 patients receiving visugromab 10 mg/kg plus nivolumab 240 mg every 2 weeks, 13 (7.5%) reported at least one grade 3 possibly treatment-related adverse event (TRAE), 1 patient (0.6%) experienced a grade 4 TRAE and 1 (0.6%) experienced a grade 5 TRAE.
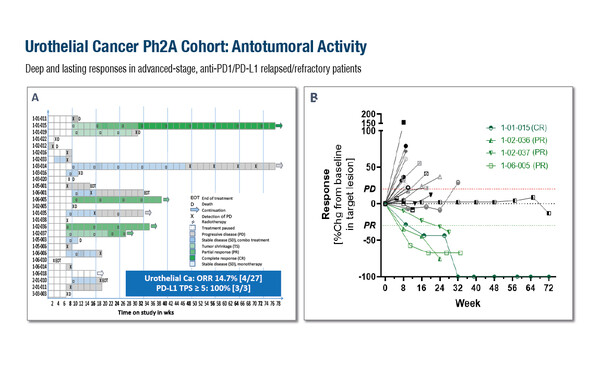
An in-silico analysis highlighted urothelial cancer and non-squamous non-small-cell lung cancer (NSCLC) as potential key GDF-15 immunosuppressed indications (among other tumour types) and mature results for these cohorts were presented. The objective response rate (ORR) was 14.7% (4/27) in patients with NSCLC, 21.1% (4/19) in non-squamous NSCLC and 14.7% (4/27) in patients with urothelial cancer, with some responses lasting more than 1 year. Responses were seen in both PD-L1-positive and -negative tumours, but notably, all tumours in the urothelial cancer cohort with PD-L1 TPS ≥5 experienced a response.
“GDF-15 is overexpressed by tumours (Proc Natl Acad Sci U S A. 2003;100:3410–3415) and its blockade appears to be a novel approach to counteract immunosuppression after checkpoint inhibition,” says Prof. Inge Marie Svane from the National Center for Cancer Immune Therapy at Copenhagen University Hospital, Herlev, Denmark. She points to recent data demonstrating that elevated serum GDF-15 levels strongly correlate with failure of PD-1-based immune checkpoint blockade therapy, but that neutralisation of GDF-15 improves both T-cell trafficking and therapy efficiency in murine models (Nat Commun. 2023;14:4253). “There do not appear to be any major safety signals at this early stage and the response rates in heavily treated patients are very encouraging,” she notes, continuing, “The durable effects in some patients indicate a need to identify biomarkers to pinpoint those patients who might benefit most from visugromab.” A phase II trial of neoadjuvant nivolumab plus visugromab for the treatment of muscle-invasive bladder cancer without prior immunotherapy is ongoing (NCT06059547). Svane also thinks that combinations of visugromab with other types of immunotherapy, for example cancer vaccines, may be an interesting avenue of investigation.
Abstract discussed:
Melero I, et al. Definitive results for NSCLC and bladder cancer cohorts in the phase 2a trial of visugromab (CTL-002) in advanced/metastatic anti-PD/PD-L1 relapsed/refractory solid tumors (GDFATHER-trial). ESMO Immuno-Oncology Congress 2023, Abstract 118O
Proffered Paper Session 1, 06.12.2023, h. 14:15 – 15:45, Room B