Pre-treatment expression of Ki-67 plus FOXC1 plus PD-L1 predicted response to neoadjuvant treatment in breast and head and neck tumours, although more work is required to determine its use in the context of immunotherapy alone
As presented at ESMO Immuno-Oncology Congress 2022 (Geneva, 7–9 December), a combined analysis of three biomarkers showed promise in predicting response to neoadjuvant use of immune checkpoint inhibitors in some solid tumours (LBA1). Researchers retrospectively analysed pre-treatment tumour expression of Ki-67, FOXC1 and PD-L1 from patients in three clinical trials of neoadjuvant therapy. The combination of the three biomarkers was used to construct biomarker-defined predicted responder (PR) and predicted non-responder (NR) groups.
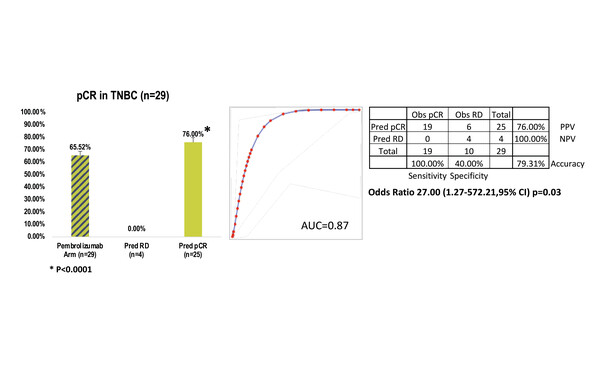
In a discovery set of patients receiving combined anthracycline, taxane and pembrolizumab for triple-negative breast cancer (TNBC), the observed response rate among 29 patients was 66%, with response rates of 76% (n=25) and 0% (n=4) in the PR and NR groups, respectively (odds ratio [OR] 27.00; 95% confidence interval [CI] 1.27–572.21; p=0.03).
The results were confirmed in two validation sets. In one set, amongst patients receiving combined anthracycline, olaparib, paclitaxel and durvalumab for TNBC, the observed response rate was 43% (n=21), with response rates of 75% (n=12) and 0% (n=9) in the PR and NR groups, respectively (OR 51.57; 95% CI 2.33–1141.00; p=0.013). In the second validation set, comprising patients with head and neck cancer receiving immunotherapy alone (nivolumab/pembrolizumab), the observed response rate was 11% (n=102), with response rates of 21% (n=38) and 5% (n=64) in the PR and NR groups, respectively (OR 5.42; 95% CI 1.34–21.92; p=0.008).
“Identifying reliable, practical biomarkers predictive of response to immunotherapy remains an area of unmet need,” says Dr Andrew Furness from the Royal Marsden NHS Foundation Trust, London, UK. “This study sought to determine whether combining the most commonly used immunotherapy predictive biomarker – PD-L1 expression – with other indicators of therapeutic response, all of which can be easily applied, might be useful in refining response prediction,” he says. “However, the positive predictive value of this approach appeared to be greater in the cohorts of patients treated with immunotherapy combined with chemotherapy compared with the patients receiving immunotherapy alone, and this raises the question of how reflective this biomarker truly is of immunotherapy response.”
Based on this, Furness believes that further retrospective/prospective analyses in patients receiving immune checkpoint inhibitor therapy alone may be useful to provide another layer of confidence that the combined biomarker approach is of potential utility.
Abstract discussed:
Ray PS, et al. Tissue-agnostic response predictor for immune checkpoint inhibitor therapy based on MKI67, FOXC1 and PDL1 expression. ESMO Immuno-Oncology Congress 2022, LBA1
Mini Oral Session 2 08.12.22, h. 14:30 –15:35, Room B. Also watch the session on the Congress virtual platform