Combination therapy with BMS-986253 shows promise in patients progressing on checkpoint inhibitors
The anti-interleukin (IL)-8 monoclonal antibody, BMS-986253 combined with nivolumab showed preliminary activity in melanoma progressing on checkpoint inhibitors, as reported in a phase I/II trial presented at ESMO Immuno-Oncology Congress 2022 (Geneva, 7–9 December) (Abstract 200MO).
BMS-986253 binds to free IL-8, preventing IL-8 signaling through the C-X-C motif chemokine receptors 1 and 2 (CXCR1 and CXCR2), which may potentially lead to reduced recruitment of polymorphonuclear myeloid-derived suppressor cells to the tumour microenvironment and improved immune-mediated lysis of tumour cells (J Immunother Cancer. 2019;7:240). Preclinical studies have shown that IL-8 blockade may make cancer cells less resistant to treatment.
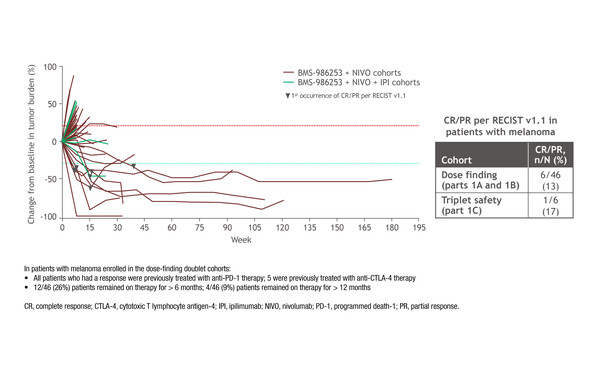
In the trial, all responders – 6 of 46 patients (13%) – had previously received anti-PD-1 therapy and 5 had received anti-CTLA-4 therapy. Around one-quarter (26%) of patients remained on therapy for more than 6 months, while 9% patients remained on therapy for more than 12 months. There was 1 complete response among 6 patients treated with BMS-986253 plus nivolumab and ipilimumab. Clinical activity with the triple combination was also observed in patients with other tumour types.
As a phase I trial, safety was the primary focus and grade ≥3 treatment-related adverse events (TRAEs) were observed in 8% of 144 patients with a range of advanced solid tumours who received BMS-986253 plus nivolumab. Two patients discontinued due to TRAEs. Grade ≥3 TRAEs were experienced by one-third of the 15 patients who received BMS-986253 plus nivolumab and ipilimumab. BMS-986253 demonstrated dose-proportional pharmacokinetics, with dose-dependent reductions in free serum IL-8 and tumour IL-8 suppression in most patients evaluated.
BMS-986253 3600 mg administered once every 2 weeks was selected as the recommended dose for the phase II part of the study, which is ongoing in approximately 200 patients with melanoma who progressed on or after anti-PD-(L)1 therapy (NCT03400332). Patients will receive BMS-986253 plus nivolumab and ipilimumab for the first 12 weeks, then BMS-986253 plus nivolumab thereafter.
Abstract discussed:
Simonelli M, et al. Anti–IL-8 BMS-986253 + nivolumab (NIVO) ± ipilimumab (IPI) in patients (pts) with advanced cancer: Update of initial phase I results. ESMO Immuno-Oncology Congress 2022, Abstract 200MO
Mini Oral Session 2 08.12.2022, h. 14:30 – 15:35, Room B. Also watch the session on the Congress virtual platform