A joint analysis of the OnCovid and ESMO-CoCARE registries confirms the protective role of anti-SARS-CoV-2 vaccination in cancer patients treated with immunotherapy
Patients exposed to immune checkpoint inhibitors (ICIs) who had received the anti-SARS-CoV-2 vaccination had reduced morbidity and mortality compared with unvaccinated patients. Confirmation of this key question in the pandemic era of oncology arises from a joint analysis of the OnCovid and ESMO-CoCARE registries, presented at ESMO Immuno-Oncology Congress 2022 (Geneva, 7–9 December), which included real-world data from 240 patients with COVID-19 who received ICIs treatment within 3 months prior to becoming infected (Abstract 237P).
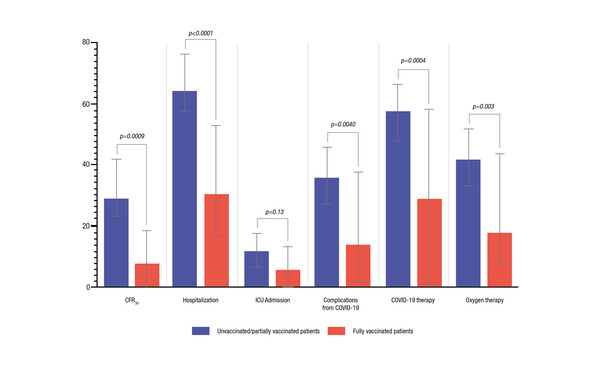
The research team found that in 42 fully vaccinated patients, the 30-day case fatality rate was 4.8% and this was significantly lower than the rate of 28.1% observed in 198 unvaccinated patients (p=0.0009). After adjustment for confounders using an inverse probability of treatment weighting (IPTW)-fitted multivariable analysis, vaccinated patients experienced a decreased risk of death at 30 days (adjusted odds ratio [aOR] 0.08; 95% confidence interval [CI] 0.01–0.69). In addition, vaccinated patients had lower rates of hospitalisation (27.5% vs 63.2%; p<0.0001), oxygen therapy (15.8% vs 41.5%; p=0.003), COVID-19 complications (11.9% vs 34.6%; p=0.004) and COVID-19-specific therapy (26.3% vs 57.9%; p=0.0004) compared with unvaccinated patients.
Since the COVID-19 outbreak, registry data have been a valuable source of information supporting oncologists to understand the impact of the viral infection on cancer treatments and how to deliver high quality care during the emergency. The analysis presented also shows a potential adaptive immune response to severe COVID-19 in patients who previously experienced immune-related adverse events (irAEs) to ICIs. The effect was explored in 38 patients (15.8%) with a history of least one irAE of any grade at a median (range) of 3.2 (0.13–48.7) months from the COVID-19 diagnosis. IrAEs were associated with a significantly decreased 30-day case fatality rate (10.8% versus 26.0%; p=0.0462), which was additionally confirmed by the IPTW-fitted multivariable analysis (aOR 0.47; 95% CI 0.33–0.67). Patients who experienced irAEs also had a higher median absolute lymphocyte count at COVID-19 diagnosis (1.4 versus 0.8×109 cells/L; p=0.0098). The authors concluded that a history of irAEs might identify patients with pre-existing protection against severe COVID-19, warranting further investigation of the adaptive immune determinants that may be responsible for this protection.
A full manuscript of the joint analysis will be published in the Journal for Immunotherapy of cancer.
Abstract discussed:
Cortellini A, et al. Immune checkpoint inhibitor therapy and outcomes from SARS-CoV-2 infection in patients with cancer: a joint analysis of OnCovid and ESMO-CoCARE registries. ESMO Immuno-Oncology Congress 2022, Abstract 237P
Poster Display 08.12.2022, h. 12:30 – 13:15, Foyer mezzanine. Also watch the session on the Congress virtual platform