Phase III trials are planned to assess combined KRAS G12C inhibition/checkpoint inhibition as first-line therapy
KRAS G12C inhibition with adagrasib plus pembrolizumab in KRAS G12C-mutated non-small-cell lung cancer (NSCLC) may be a beneficial first-line approach, according to a presentation at ESMO Immuno-Oncology Congress 2022 (Geneva, 7–9 December) (LBA4).
Historically, targeting KRAS alterations in NSCLC has proved difficult, however, specific KRASG12C covalent inhibitors have now been developed (N Engl J Med. 2022;387:180–183). Recently, efficacy was demonstrated with the irreversible KRAS G12C inhibitor, adagrasib, in patients with previously treated KRAS G12C-mutated NSCLC in the phase I/II KRYSTAL-1 trial (N Engl J Med. 2022;387:120–131). Preclinical studies indicated that adagrasib may recondition the tumour immune microenvironment and sensitise tumours to checkpoint inhibitor therapy (Mol Cancer Ther. 2021;20:975–985), leading to the rationale to assess the effects of adagrasib plus pembrolizumab in a cohort of KRYSTAL-1 and also as part of the dedicated first-line KRYSTAL-7 phase II trial of adagrasib as monotherapy or in combination with pembrolizumab (NCT04613596).
As presented at the Congress, 7 patients from a phase Ib cohort of KRYSTAL-1 received first-line adagrasib plus pembrolizumab and among these patients, an objective response rate (ORR) of 57% and a disease control rate (DCR) of 100% were reported, with a median follow-up of 19.3 months. All 4 patients who responded had a durable response of more than 9 months, with 2 continuing to receive treatment beyond 18 months.
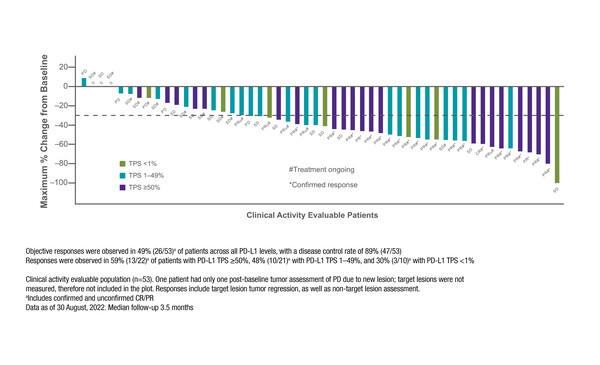
In KRYSTAL-7, adagrasib plus pembrolizumab was associated with an ORR of 49% and a DCR of 89% in a preliminary efficacy analysis of 53 evaluable treatment-naïve patients, with a median follow-up of 3.5 months. Treatment was ongoing in 66% of patients, including in 24 patients who had a response to therapy.
In terms of the safety of the combination, grade 3 treatment-related adverse events (TRAEs) occurred in 40% of patients in KRYSTAL-7 and were most commonly early elevations in aspartate aminotransferase (9%) or alanine aminotransferase (8%). In total, 4% of patients experienced grade 4 TRAEs, with no grade 5 TRAEs. TRAEs led to adagrasib dose reduction in 31% of patients and to dose interruption in 41% of patients. TRAEs led to discontinuation of both agents in 3% of patients and of pembrolizumab only in 3% of patients, with no TRAE-related discontinuations of adagrasib only. The safety profile in the KRYSTAL-1 cohort was consistent with these findings.
“There has been increasing concern about the toxicity profile of combining a KRAS G12C inhibitor with immune checkpoint inhibitors. Data presented earlier this year have shown a grade 3 effect with sotorasib in combination with immunotherapy up to 74% with up to 50% discontinuation rate (J Thorac Oncol. 2022. Issue 9, Supplement, s10-s11, September 1)", says Dr Alfredo Addeo, Geneva University Hospital, Switzerland. "The preliminary data on safety presented at ESMO Immuno-Oncology Congress 2022 are reassuring. The combination has shown a manageable safety profile consistent with either agent as monotherapy, and a low discontinuation rate.”
Based on the results, phase III trials are planned in first-line NSCLC, evaluating concurrent adagrasib 400 mg twice daily plus pembrolizumab versus standard-of-care by PD-L1 status.
Abstract discussed:
Jänne PA, et al. Preliminary safety and efficacy of adagrasib with pembrolizumab in treatment-naïve patients with advanced non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. ESMO Immuno-Oncology Congress 2022, LBA4
Proffered Paper Session 07.12.2022, h. 14:05 – 15:40, Room B. Also watch the session on the Congress virtual platform