Data suggest that assessing the quality as well as the quantity of tumour mutations may enhance prediction of outcomes in patients treated with immune checkpoint inhibitors
Results from a post hoc analysis presented today at the ESMO Immuno-Oncology Congress 2021 demonstrated that in patients with advanced cancer and high tumour mutational burden (TMB), mutations in certain genes were associated with short overall survival (OS), while others were protective, providing important insights on outcome beyond TMB alone (Abstract 40P).
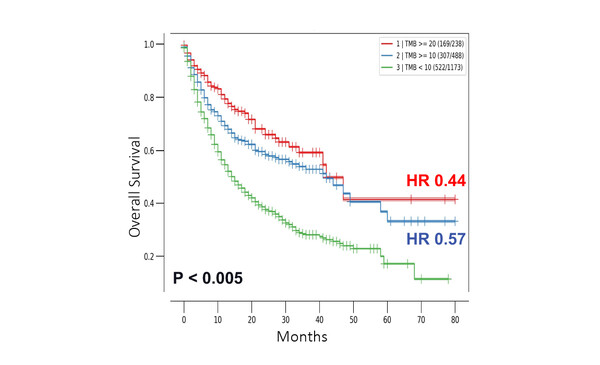
Clinical data from 1,662 patients with advanced cancer treated with checkpoint inhibitors were retrieved from the MSK-IMPACT cohort available on the cBioPortal for Cancer Genomics – an open-source platform that provides intuitive visualisation and analysis of large-scale cancer genomics data sets. When TMB was analysed, with a maximum follow-up of 80 months, median OS was 42 months for both TMB ≥20 mut/Mb and ≥10 mut/Mb, and 15 months for TMB <10 mut/Mb (p<0.005). Risk of death was also significantly lower in patients with TMB ≥20 mut/Mb (hazard ratio [HR] 0.44; 95% confidence interval [CI] 0.34–0.56; p<0.005) and TMB ≥10 mut/Mb (HR 0.57; 95% CI 0.49–0.67; p<0.005) versus TMB <10 mut/Mb (Figure).
“When research began on TMB we had high hopes for its use as a predictive biomarker to select patients likely to respond well to immune checkpoint inhibitors,” says Prof. Christian Rolfo, Associate Director for Clinical Research at the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai, New York, NY, USA. “Data from retrospective analyses such as these and from non-randomised trials are promising, but insufficient predictive power was observed with TMB in prospective trials, for example, in non-small-cell lung cancer (NSCLC).” He also thinks that issues over the best methods and cutoffs have made standardisation and application of TMB in clinical practice very difficult.
In the second part of the presented analysis, genomic profiling was combined with TMB. Findings show that in patients with high TMB (≥10 mut/Mb), the risk of death was greater in patients with mutations in STK11 (HR 2.96; p<0.0007) and TP53 (HR 1.89; p<0.0032), while mutations in TERT (HR 0.54; p<0.0045) and NOTCH3 (HR 0.26; p<0.0355) were protective. “These data suggest that assessing TMB as a number is not enough and that integrating information on the quality of the mutations can add real value to TMB. Seeing the effect on outcomes of the presence of mutations in STK11 or NOTCH3 in patients with high TMB is particularly informative,” says Rolfo. These data further reinforce previous findings suggesting that TMB alone might not be informative of benefit from immune checkpoint blockade. Some tumours, such as colorectal cancer, seem influenced by microsatellite instability (N Engl J Med. 2021;384:1168–1170), while certain solid tumours are affected by CD8 T-cell levels (Ann Oncol. 2021;32:661–672). Other researchers are exploring the combination of TMB with different genetic analyses and neoantigen-related interplay with good effect. “Results from a recent study suggest that loss of heterozygosity of human leukocyte antigen class I has the potential to further refine TMB and lead to better stratification (Cancer Discov. 2021;11:282–292),” he concludes. “Prospective data are needed to confirm the benefits of combining TMB with other genetic analyses. Only then will we know if there is hope for a ‘Lazarus effect’ to resurrect the use of TMB.”
Xavier CB, et al. Interplay between mutational profile and TMB on overall survival: a posthoc analysis of 1,662 metastatic patients treated with immune checkpoint inhibitors. ESMO Immuno-Oncology Congress 2021, Abstract 40P
On-Demand e-Poster Display, Congress virtual platform