Immunotherapy plus chemotherapy as first-line treatment choice for advanced disease without oncogene mutations, irrespective of ethnicity and PD-L1 status
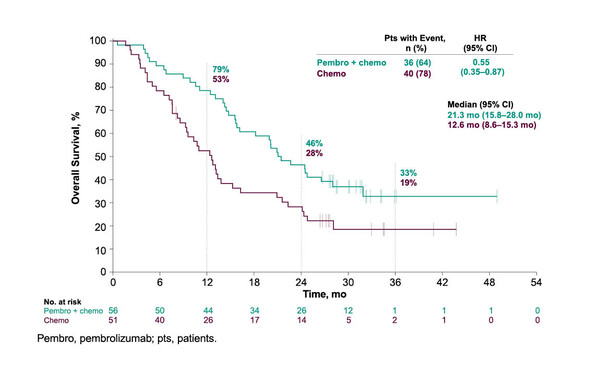
A pooled analysis including 107 Asian patients with advanced/metastatic non-small-cell lung cancer (NSCLC) who were enrolled in KEYNOTE-021 cohort G (NCT02039674), KEYNOTE-407 (NCT02775435 and NCT03875092) and KEYNOTE-189 (NCT02578680 and NCT03950674) report that the addition of pembrolizumab to chemotherapy was associated with an 8.7-month improvement in overall survival (OS) compared with chemotherapy alone (median 21.3 versus 12.6 months; hazard ratio [HR] 0.55; 95% confidence interval [CI] 0.35–0.87) and a 2.4-month improvement in progression-free survival (8.4 versus 6.0 months; HR 0.64; 95% CI 0.43–0.96) (Abstract 6P).
“These findings confirm that the benefits of first-line chemotherapy plus pembrolizumab in NSCLC are not ethnicity specific,” says Dr Alfredo Addeo from Geneva University Hospital, Switzerland, commenting on the data presented at the ESMO Immuno-Oncology Congress 2021. “Given the potential for ethnicity to impact on the clinical activity of oncology therapies, the results provide reassurance that pembrolizumab is as effective in East Asian patients as it is in Western populations.”
In the analysis, objective response rates were 71.4% with pembrolizumab plus chemotherapy and 43.1% with chemotherapy alone. Corresponding median durations of response were 6.7 months and 4.9 months.
All East Asian patients had a PD-L1 tumour proportion score <1%. Chemotherapy comprised platinum-pemetrexed for non-squamous NSCLC and carboplatin-paclitaxel/nab-paclitaxel for squamous NSCLC. Grade ≥3 adverse events occurred in 80.4% of patients receiving pembrolizumab plus chemotherapy and in 82.4% receiving chemotherapy alone. Among 18 patients who crossed over from chemotherapy alone to pembrolizumab plus chemotherapy during the study, the median OS from pembrolizumab initiation was 11.7 months. Nine patients receiving pembrolizumab plus chemotherapy completing 35 cycles of pembrolizumab had partial responses.
“Data presented support results from a previous pooled analysis, demonstrating the clinical benefits of pembrolizumab plus chemotherapy in PD-L1-negative advanced NSCLC in patients of different ethnicities,” concludes Addeo (Cancer. 2020;126:4867–4877). “Combining the findings from this analysis with results from a variety of different clinical studies with immune checkpoint inhibitors, the overall take-home message is that immunotherapy should form part of first-line treatment for NSCLC without oncogene mutations in patients of all ethnicities and irrespective of PD-L1 expression levels.”
Cheng Y, et al. Pembrolizumab plus chemotherapy vs chemotherapy in Asian patients with PD-L1 negative advanced NSCLC: Pooled analysis of KN021G, KN189 and KN407. ESMO Immuno-Oncology Congress 2021, Abstract 6P
On-Demand e-Poster Display, Congress virtual platform