Sub-group analyses of CheckMate 227 part 1 provide reassurance but no definite guidance regarding standard treatment for different patient sub-groups
The CheckMate 227 part 1 trial previously reported that first-line nivolumab plus ipilimumab prolonged overall survival (OS) versus chemotherapy in patients with stage IV recurrent NSCLC, no known EGFR/ALK alterations and PD-L1 ≥1% (primary endpoint) or <1% (N Engl J Med. 2019;381:2020–2031), with durable efficacy at the 4-year follow-up (J Thorac Oncol. 2021;Oct 11. Online ahead of print). Two presentations today at the ESMO Immuno-Oncology Congress 2021 report data from sub-group analyses of the trial, with a minimum follow-up of 4 years.
In an exploratory analysis, nivolumab plus ipilimumab improved median OS compared with chemotherapy in 475 patients with non-squamous NSCLC and evaluable mutations, including KRAS (34%), STK11 (16%) and KEAP1 (8%) (20.2 versus 16.3 months; hazard ratio [HR] 0.75; 95% confidence interval [CI] 0.61–0.93; 4-year OS rates, 32% versus 20%) (Abstract 4O). Similar survival improvements were seen in patients with and without mutations in KRAS (HR 0.79 and 0.73, respectively) and STK11 (HR 0.78 and 0.75, respectively) and without mutations in KEAP1 (HR 0.80). Interpretation of the HR of 0.31 among patients with KEAP1 mutations is limited by small patient numbers. In the chemotherapy arm, median OS was lower among patients with versus without mutations in the STK11 (11.2 versus 18.5 months) and KEAP1 (8.9 versus 16.7 months) sub-groups, but not in the KRAS sub-group (15.7 versus 17.9 months).
We now have a range of treatment strategies for advanced NSCLC and the challenge is to find biomarkers that will help clinicians differentiate between the different approaches for each of their patients.
Professor Benjamin Besse from Institut Gustave Roussy, Villejuif, France, explains: “We already know that STK11 and KEAP1 mutations are associated with a poor prognosis, a finding that was confirmed by this analysis. However, none of the mutations investigated in this analysis were shown to be predictive of response to nivolumab plus ipilimumab.” With the caveat that the patient numbers in some sub-groups are small, he continues, “The results will not have a practice-changing impact, but they do confirm that this treatment option is valid across a variety of tumour mutations.”
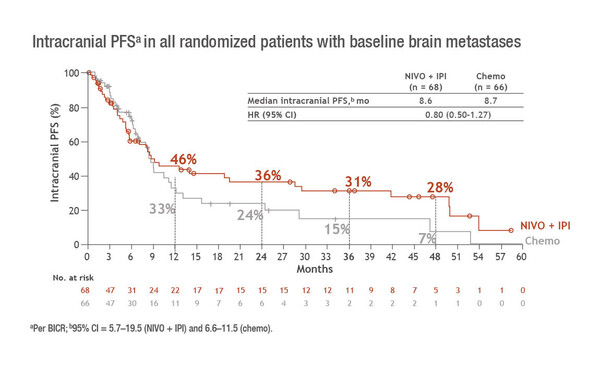
A second sub-group analysis of CheckMate 227 presented at the Congress confirmed that OS benefits of nivolumab plus ipilimumab over chemotherapy were similar in patients with and without treated asymptomatic brain metastases, as previously reported in a post-hoc analysis (Cancer Res. 2020;80(Suppl 16). Abs. CT221). Results from a 4-year follow-up show OS HRs of 0.63 (95% CI 0.43–0.92) and 0.75 (95% CI 0.65–0.86) in patients with (n=134) and without (n=1,032) brain metastases, respectively (Abstract 122MO). Among the patients with brain metastases – which are a common complication of NSCLC – the median intracranial progression-free survival (PFS) was 8.6 months with nivolumab plus ipilimumab versus 8.7 months with chemotherapy (HR 0.80; 95% CI 0.50–1.27) (Figure). Corresponding 4-year PFS rates were 28% and 7%. The intracranial objective response rate was the same in both treatment arms (29%), but the duration of response was 45.5 months with nivolumab plus ipilimumab and 32.0 months with chemotherapy. Neurological treatment-related adverse events (all grade 1/2) were seen in 16% of patients receiving nivolumab plus ipilimumab and 17% of those receiving chemotherapy.
“Given the potential for immunotherapy to increase inflammation and exacerbate oedema, it is reassuring to see that there was no increase in adverse events in the central nervous system with nivolumab plus ipilimumab compared with chemotherapy, although we must remember that this was a selected patient population and does not represent all-comers,” says Besse.
Addressing both sub-group analyses, Besse draws attention to the fact that the chemotherapy comparator arm in CheckMate 227 is no longer standard of care. “In this respect, while both analyses provide useful information, neither helps to define the best treatment approach for the patients involved. It would be interesting to see if similar analyses with chemotherapy plus immunotherapy – from the third arm of the CheckMate 227 part 1 trial in patients with PD-L1 <1% and from the CheckMate 9LA study (Lancet Oncol. 2021;22:198–211) – could help to further refine treatment decisions,” he says.
Ramalingam SS, et al. Nivolumab (NIVO) + ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced NSCLC (aNSCLC) in CheckMate 227 Part 1: efficacy by KRAS, STK11, and KEAP1 mutation status. ESMO Immuno-Oncology Congress 2021, Abstract 4O
Proffered Paper Session 1 09.12.2021, h. 09:00 – 10:30, Room A
Reck M, et al. Nivolumab (NIVO) + ipilimumab (IPI) as first-line (1L) treatment (tx) for patients (pts) with advanced NSCLC (aNSCLC) and baseline (BL) brain metastases (mets): intracranial and systemic outcomes from Checkmate 227 Part 1. ESMO Immuno-Oncology Congress 2021, Abstract 122MO
Mini Oral Session 09.12.2021, h. 11:00 – 12:40, Room C