A study associates reductions in ctDNA with major pathologic response following neoadjuvant atezolizumab in resectable stage IB–IIIB NSCLC, but clinical application is still some way off
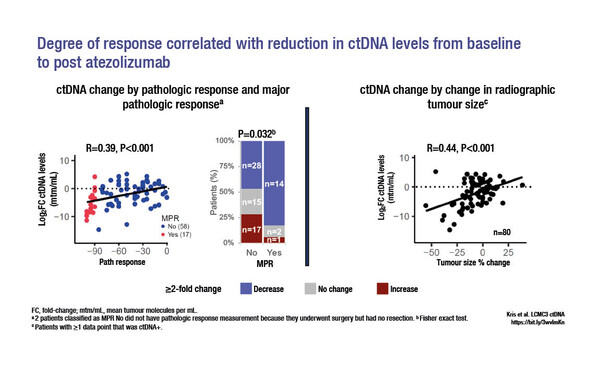
Results from a study presented today at the ESMO Immuno-Oncology Congress 2021 demonstrated that reductions in post-atezolizumab circulating tumour (ct)DNA levels were greater in non-small cell lung cancer (NSCLC) patients who achieved a major pathological response (MPR) compared with those not achieving an MPR (primary endpoint: median log2 fold change −4.8 versus 0.3, p<0.001) (Abstract 1O).
The study profiled ctDNA from patients enrolled in the Lung Cancer Mutation Consortium 3 trial, which earlier this year demonstrated that neoadjuvant atezolizumab monotherapy in resectable NSCLC led to an MPR in 20% of patients (J Thorac Oncol. 2021;16(Suppl)S59–S61). Altogether, 106 (84%) of 126 patients with sufficient test tissue had tumour variants suitable for monitoring. ctDNA was detected in 72% of samples (n=104) pre atezolizumab, 56% (n=102) post atezolizumab and 12% (n=51) post-surgery. Median (range) ctDNA levels decreased from 3 (0–4448) mean tumour molecules/mL plasma (mtm/mL) pre atezolizumab to 0.5 (0–406) mtm/mL post atezolizumab and 0 (0–35) mtm/mL post surgery (pre atezolizumab vs post atezolizumab, p<0.01 and all other paired comparisons p<0.001).
Post-atezolizumab reductions in ctDNA levels were associated with pathologic response (p<0.001, r=0.39) and a reduction in radiographic tumour size (p<0.001, r=0.44) (Figure). The 2-year disease-free survival (DFS) rate was 75% for patients who were ctDNA negative post-surgery and 50% for those who were ctDNA positive (hazard ratio 3.6; 95% confidence interval 1.12–1.56; p=0.20).
These results add to the growing bank of knowledge regarding ctDNA use in the neoadjuvant treatment of NSCLC, but we need to have a better understanding of the implications of ctDNA findings
Assistant Prof. Lizza Hendriks from Maastricht University Medical Center+, Netherlands, comments on the findings: “Although this analysis shows that ctDNA clearance is associated with a higher chance of achieving an MPR – and, subsequently, improved DFS – it is necessary to determine if the information provided by ctDNA adds value to predicting outcome, or if MPR alone is sufficient.”
According to Hendriks, the role of ctDNA in adapting management strategies also requires more clarification. “If a neoadjuvant approach leads to ctDNA clearance, do those patients still require adjuvant therapy? And should patients who do not achieve ctDNA clearance with neoadjuvant therapy be changed to a different regimen in the adjuvant setting? We just don’t have the data,” she says, highlighting that a significant drawback of the technique is the fact that not all patients have detectable pre-treatment ctDNA. “For a complete picture, it is really important that studies report the proportions of patients who are ctDNA negative at baseline, and those who are not test evaluable, together with their outcomes,” she says.
In addition to the challenges surrounding data interpretation, there are technical limitations to the utility of ctDNA. These include the use of different tests with varying sensitivities, which hinder interpretation of results between trials, and the requirement by some tests for tumour tissue in addition to the liquid biopsy. “In time, and with further validation, ctDNA could become a marker for guiding some oncology treatment choices for patients, but we are not there yet,” concludes Hendriks.
Kris MG, et al. Dynamic circulating tumour DNA (ctDNA) response to neoadjuvant (NA) atezolizumab (atezo) and surgery (surg) and association with outcomes in patients (pts) with NSCLC. ESMO Immuno-Oncology Congress 2021, Abstract 1O
Proffered Paper Session 1 09.12.2021, h. 09:00 – 10:30, Room A