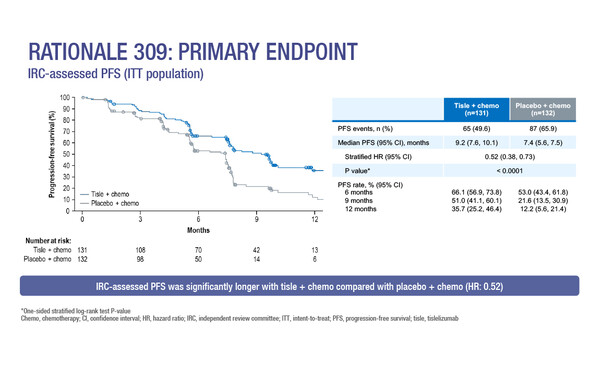
Tislelizumab in combination with gemcitabine and cisplatin prolonged PFS compared with chemotherapy alone in the RATIONALE 309 study
New combination therapies might be a useful way to tackle the biological heterogeneity of advanced head and neck cancers and improve outcomes with immunotherapy, which are relatively poor compared with other carcinomas of the squamous cell type. At the ESMO Immuno-Oncology Congress 2021, promising results from the RATIONALE 309 trial showed that the addition of antibodies against PD-1 appears to improve the efficacy of the standard chemotherapy regimen (Abstract 121O).
In the randomised, global, double-blind, phase III trial, the anti-PD-1 antibody, tislelizumab, in combination with standard-of-care gemcitabine and cisplatin, improved progression-free survival (PFS) in the first-line treatment for recurrent/metastatic nasopharyngeal cancer (NPC). Median independent review committee-assessed PFS was 9.2 months with tislelizumab plus gemcitabine-cisplatin and 7.4 months with placebo plus chemotherapy (hazard ratio [HR] 0.52; 95% confidence interval [CI] 0.38–0.73; p<0.0001). Objective response rates (ORRs) were 69.5% and 55.3%, respectively, with 16.0% and 6.8% patients achieving a complete response.
“The clear improvement in PFS seen with tislelizumab is consistent with that observed in similarly designed phase III trials with camrelizumab (Lancet Oncol. 2021;22:1162–1174) and with toripalimab (Nat Med. 2021;27:1536–1543),” says Prof. Lisa Licitra from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy, noting that mature overall survival (OS) data are still needed to confirm efficacy in all three trials.
Licitra also highlights the importance of timings of such effects. “Separation of the PFS curves appears to occur after the chemotherapy cycles, which may indicate that immunotherapy is only needed in the maintenance phase, and this warrants further study. If we find that immunotherapy maintenance is the main driver of the PFS separation, this may provide evidence in favour of investigating immunotherapies in the adjuvant setting to improve survival,” she says.
Advanced head and neck carcinomas are difficult-to-treat malignancies and heterogeneity may be at least partly responsible for this. Despite being the same histotype, there is in fact diversity between squamous cell tissue sites such as lung, cervix and head and neck with regard to alterations in chromosomes, DNA methylation, messenger and microRNA expression and mutations (Cell Rep. 2018;23:194–212). In addition, within squamous cell carcinomas of the head and neck (SCCHN), there is also additional major differentiation due to human papillomavirus (HPV) infection and varying levels of immune cell infiltration (Ann Oncol. 2016;27:1675–1685).
Monalizumab is a monoclonal antibody that targets the inhibitory natural killer group 2A (NKG2A) receptor localised on natural killer (NK) and T cells (Cell. 2018;175:1731–1743). It is thought that monalizumab may enhance NK and T cell activity in SCCHN where NKG2A’s ligand, human leukocyte antigen E, is overexpressed; however, results with monalizumab as monotherapy in patients progressing on prior therapy have been disappointing (Eur J Cancer. 2021;158:17–26).
In a Mini Oral Session, preliminary findings were presented from a phase II trial investigating monalizumab, in combination with cetuximab and durvalumab, in the first-line treatment of recurrent/metastatic SCCHN (Abstract 123MO). After a median follow-up of 16.3 months, ORR was 32.5%, including three complete responses, while median PFS was 6.9 months, and 12-month OS was 59%.
Cautioning that this was a small study involving 40 patients only, Licitra suggests that the initial overall results do not seem markedly better with the triple combination compared with pembrolizumab and chemotherapy (Lancet. 2019;394:1915–1928). “If response occurred regardless of PD-L1 expression, the triple combination may be promising; however, we cannot conclude that at the moment and we await further evaluation,” she says.
Yang Y, et al. RATIONALE 309: A randomized, global, double-blind, Phase 3 trial of tislelizumab (TIS) vs placebo, plus gemcitabine + cisplatin (GP), as 1L treatment for recurrent/metastatic nasopharyngeal cancer (RM-NPC). ESMO Immuno-Oncology Congress 2021, Abstract 121O
Proffered Paper Session 2, 10.12.2021, h. 09:00 – 10:30, Congress virtual platform
Colevas DA, et al. Monalizumab, cetuximab and durvalumab in first line treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): a phase 2 trial. ESMO Immuno-Oncology Congress 2021, Abstract 123MO
Mini Oral Session, 09.12.2021, h. 11:00 – 12:40, Congress virtual platform