Early clinical findings with the anti-claudin-6 CAR-T-cell product BNT211, with or without amplifying vaccine, in patients with refractory solid tumours are promising but more data are required
A novel approach combining engineered cells and the chimeric antigen receptor (CAR)-T cell-amplifying RNA vaccine (CARVac) shows promise in expanding persistence and functionality of CAR-T cell therapy in advanced solid tumours, according to early results from a phase I trial presented as a late-breaking abstract at the ESMO Immuno-Oncology Congress 2021 (Abstract LBA1).
In the study, CARVac was administered to patients in combination with BNT211, a CAR-T-cell product candidate that targets the tumour antigen claudin-6. “The idea is backed by strong preclinical data which show that the vaccine promotes CAR-T cell stimulation in vivo (Science. 2020;367:446–453),” says Prof. Sebastian Kobold from Klinikum der Universität München, Germany. “Also, the choice of claudin-6 as a novel target is based on the fact that it is an oncofoetal protein and is therefore, at least theoretically, tumour specific,” he adds.
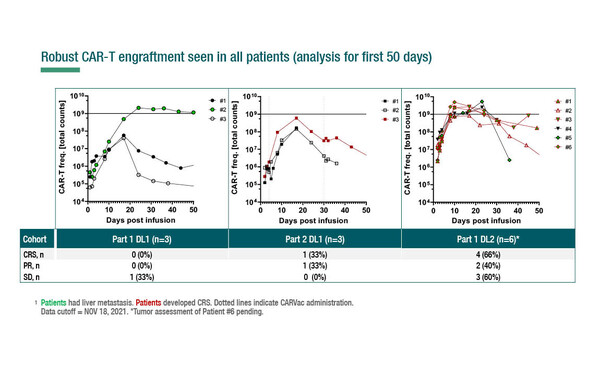
Among 15 patients, all with claudin-6-positive relapsed or refractory disease, one dose-limiting toxicity (DLT, prolonged pancytopenia) was reported, and 13 patients had grade ≥3 adverse events, with 22 grade ≥3 events suspected to be related to treatment. The trial used a 3+3 design with separate BNT211 dose escalations for monotherapy (part 1) and its combination with 3-weekly CARVac (part 2). Four patients receiving monotherapy at dose level 2 in part 1 and 3 patients receiving combination therapy (n=1 at dose level 1 and n=2 at dose level 2) in part 2 had manageable cytokine release syndrome (grade 1 to 2), accompanied by high interleukin-6 levels and with no signs of neurotoxicity. CARVac administration led to transient flu-like symptoms that resolved within 24 hours. Peripheral blood CAR-T-cell frequency revealed effective engraftment, followed by a decline after Day 17 (Figure).
Authors reported 4 cases of partial response, an additional 4 cases of stable disease with shrinkage of target lesions, and 1 patient showing no signs of clinical activity. Notably, 3 of the 4 partial responses were achieved in patients with testicular cancer who had all relapsed on recent high-dose chemotherapy.
Kobold thinks that the approach is a promising way to overcome some of the problems with the use of CAR-T cells in solid tumours. “Although CAR-T cells have shown good efficacy in haematological cancers, their use in solid tumours has been limited by a number of factors, including the identification of a suitable target, restricted entry of CAR-T cells into tumour tissue and the immunosuppressive nature of the tumour microenvironment,” he explains.
The lack of unexpected toxicities or DLTs and the presence of signals suggesting at least transient clinical response in patients with relapsed or refractory solid tumours are encouraging. “However, only a few patients have been treated in this phase I trial so far. It will be interesting to see efficacy data with the BNT211 CARVac combination in more patients,” he concludes.
Haanen JBAG, et al. BNT211: A Phase I/II trial to evaluate safety and efficacy of CLDN6 CAR-T cells and CARVac-mediated in vivo expansion in patients with CLDN6-positive advanced solid tumor. ESMO Immuno-Oncology Congress 2021, Abstract LBA1
Mini Oral session 9.12.2021, h. 11:00 – 12:40, Room C