Preliminary results of NW-301V are promising, with a lack of severe adverse effects at all doses tested in patients with pancreatic cancer or colorectal cancer
Encouraging safety and efficacy data were presented at the ESMO Congress 2025 (Berlin, 17–21 October) from the first-in-human phase I study of NW-301V, an autologous T-cell receptor-engineered T-cell therapy (TCR-T) targeting mutant KRAS G12V in advanced solid tumours (Abstract 1514O).
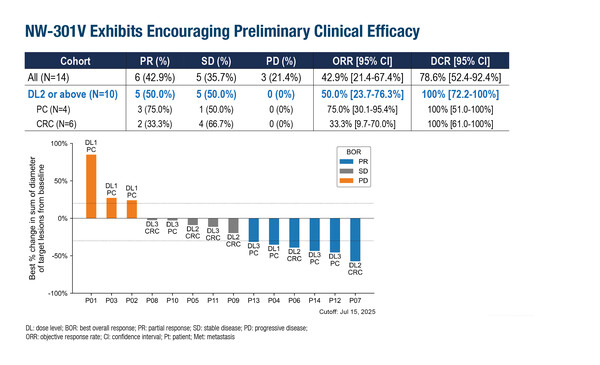
Three dose levels of NW-301V were evaluated in 14 patients with unresectable or advanced pancreatic or colorectal cancer positive for HLA-A*11:01 and KRAS G12V, who had no alternative treatment options. The most common grade ≥3 adverse events were decreased lymphocytes (78.6%), decreased white blood cells (50.0%) and decreased neutrophils (50.0%); all related to lymphodepletion. Grade 1–2 cytokine release syndrome (CRS) was reported in 6 (42.9%) patients and there were no occurrences of immune effector cell-associated neurotoxicity syndrome (ICANS). Preliminary efficacy with NW-301V was positive, with an objective response rate of 42.9% for the overall study population. Across 10 patients who received target dose levels two and three (1.5 x 109 and 7.5 x 109 cells, respectively), 50% achieved a partial response and 50% achieved stable disease, with median progression-free survival of 5.8 months.
“The aim of a phase I study is usually to establish the toxicity profile and less about determining efficacy, but the extent of the activity seen with NW-301V is encouraging and demonstrates a potentially potent therapy,” says Dr Jonathan Lim from The University of Manchester and The Christie NHS Foundation Trust, Manchester, UK. Patients in the study had received a median of three prior lines of systemic therapy, which Lim notes make these results in a heavily pre-treated population even more remarkable. Lim adds, “I am particularly heartened by the lack of serious side-effects at the doses tested. All the CRS events reported – which we often see with T-cell-based therapies – were grade 1–2, meaning they are relatively straightforward to control medically.”
Recent advances against the KRAS G12C mutation provide hope for the development of much-needed therapies for G12V, which is a well-established driver mutation in many pancreatic and colorectal cancers (NPJ Precis Oncol. 2022;6:91). KRAS mutations can weaken tumour-infiltrating T cell function and create an immunosuppressive tumour microenvironment (Front Immunol. 2025;16:1509173), making TCR-T therapy a potentially advantageous approach. However, there are challenges to overcome: “There can be a long lead-time for the manufacture of TCR-T and the current high cost could prohibit broad use in clinical practice,” cautions Lim. Additionally, the specificity of NW-301V for the G12V mutation alone could also be restrictive. A recent study of an autologous neoantigen-specific T-cell therapy was generated to display specificity toward multiple mutants in patients with metastatic melanoma (Nat Med. 2025;31:881–893), which may be advantageous considering the intra- and inter-tumoural heterogeneity often seen in solid tumours. Nevertheless, Lim is optimistic for the future of NW-301V, concluding, “Further trials would be eagerly awaited considering the response rate seen in this small sample size, and it would be interesting to test NW-301V as an earlier line of therapy. Patient selection will be critical given the logistic and cost issues.”
Programme details:
Bai X, et al. First-in-human phase I study of TCR-T therapy targeting KRAS G12V in metastatic solid tumors. ESMO Congress 2025 - Abstract 1514O