Positive signals from early-phase studies suggest that newer molecular technologies may help to disclose the promises of T-cell therapies in different settings
At the ESMO Congress 2023 (Madrid, 20–24 October), presentations of results from early-phase T-cell therapy-based trials reflect the innovative development that has occurred in the field in recent years, leading to new promise for some difficult-to-treat cancers, such as ovarian, oesophageal and metastatic colorectal cancers (mCRC).
“After years of negative studies and high rates of toxicity, newer molecular technologies and bioinformatic platforms appear to have helped give rise to enhanced cell therapies with more positive results, thanks to the improved design of chimeric antigen receptors, upregulated specificity and perhaps down-regulation of off-tumour activity,” says Prof. Inge Marie Svane from the National Center for Cancer Immune Therapy at Copenhagen University Hospital, Herlev, Denmark, commenting on the four studies presented.
In the first study, a phase I/IIa dose escalation trial of claudin-6 (CLDN6)-targeting chimeric antigen receptor (CAR)-T cells with or without a CLDN6-encoding CAR-T cell-amplifying RNA vaccine (CARVac), CAR-T cells were generated via an automated, scalable production process as opposed to manually (LBA35). The results in 28 efficacy-evaluable, heavily pre-treated patients with various cancers, including epithelial ovarian carcinoma and testicular germ-cell tumours, demonstrated partial responses in 9 patients and stable disease in a further 9 patients. Of 19 patients who received a dose of ≥1 x 108 CAR-T cells, there were 8 with partial responses and 8 with stable disease, giving an overall response rate of 42% and disease control rate of 84%. Safety data confirmed that the automatically manufactured CLDN6 CAR-T cells had a similar safety profile to manually produced cells, with 23 patients (61%) reporting treatment-emergent adverse events of grade 3 or above related to CAR-T cells or combination treatment. “I am not surprised that the results for the automatically manufactured CAR-T cells are similar to those of the manually-derived cells considering the great progress of technology platforms made in recent years,” says Svane, adding, “These results confirm a good efficacy response in difficult-to-treat cancers.”
A second presentation featured interim results from a phase I, first-in-human study of the non-engineered neoantigen-specific T-cell product (BNT221) in treatment-resistant metastatic melanoma (Abstract 1017O). “This is a new, interesting strategy that uses peripheral blood mononuclear cells collected via leukapheresis from individual patients to create personalised BNT221, which contains T-cell responses targeting multiple neoantigens specific to a patient’s tumour,” says Svane. “Collection of T cells from blood rather than via surgical means makes it easy to obtain samples,” she adds. The immunogenic neoantigen product for each patient was predicted using a bioinformatics platform and was then used to prime, activate and expand memory and de novo T-cell responses from both CD4+ and CD8+ compartments in an ex vivo induction process. Nine patients received a single infusion of BNT221 after lymphodepleting chemotherapy, with no observed dose-limiting toxicities, and post-infusion grade 3–4 haematological toxicities associated with lymphodepletion. There was tumour shrinkage detected in 4 of the 9 patients. A patient who showed the best response to therapy had a reduction in target and non-target lesions, accompanied by increased frequency of neoantigen specific T cells in the peripheral blood and tumour. Stable disease (SD) lasting from 5 to 35+ weeks was reported in 1 patient. There was evidence of tumour infiltration in 1 patient tested. “I think it is important to test the strategy of using a broad range of antigenic targets rather than a single target,” says Svane. “While only a small number of patients have been treated so far, making it too early to predict the extent or durability of responses, I think it is encouraging that the infused new antigen-specific T cells infiltrated the tumour lesion, meaning they are capable of homing in on the tumour.”
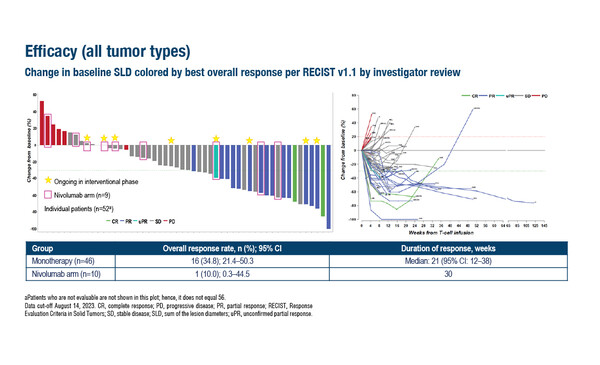
Also presented at the Congress was the larger phase I SURPASS study in which 56 patients with a range of unresectable/metastatic, pre-treated solid tumours received ADP-A2M4CD8, T-cell receptor (TCR) T-cell therapy targeting MAGE-A4 and modified with a CD8α coreceptor, as monotherapy or in combination with nivolumab (Abstract 1019O). The overall response rate according to investigator reviewed RECIST 1.1 criteria was 34.8% in 46 patients who received monotherapy, including 2 complete responses and 14 partial responses. The median duration of response was 21 weeks (95% confidence interval [CI] 12–38). For the 10 patients who received ADP-A2M4CD8 + nivolumab, the overall response rate was 10.0% and the median duration of response was 30 weeks.
Commenting on these results, Svane says, “The response rates are very encouraging, and what is important is that patients with different cancer types are included in the study – not just melanoma, but also ovarian and oesophageal cancers, which are usually not very accessible to immunotherapy. It would be interesting to know the responses in the different cancer types. However, the durability of response could be an issue, as the median response was just over 20 weeks, which could be enhanced with the addition of the checkpoint inhibitor.”
Finally, an ongoing phase I, open-label, dose-escalation study of the guanylyl cyclase 2C (GC-C)-targeted CAR-T cell therapy, IM96, is evaluating its safety and efficacy in patients with CRC who had failed ≥3 lines of therapy (Abstract 1018O). GC-C is a transmembrane receptor expressed on the surface of intestinal epithelial cells, the expression of which is retained both in the primary tumour and metastatic cells, therefore making it a logical target for cancer therapy. Among 9 patients who received a single infusion of IM96, grade 1–2 cytokine release syndrome occurred in 6 patients (66.7%) with dramatic increase of interleukin-6 reported in 5 of these patients. Three different doses of IM96 were administered and any-grade diarrhoea and any-grade oral mucositis (5/9 patients [55.6%] and 3/9 patients [33.3%], respectively) were mostly reported in patients receiving the intermediate and higher IM96 doses (6 × 108 and 12 × 108 CAR-T cells). Dose-limiting toxicity was not reported. The disease control rate was 100% among patients with moderate-to-strong GC-C expression in ≥30% of tumour cells. The tumours of all patients who received an IM96 dose of ≥6 × 108 CAR-T cells reduced in size. CAR-T cells proliferated in all patients, peaking 7–14 days after infusion.
“GC-C appears to be a good target in CRC as it is only expressed in intestinal cells – and over-expressed in cancerous cells – but of course you also have to think of intestinal side-effects,” says Svane. “The frequency of grade 3 diarrhoea reported in this study appears acceptable, suggesting that the off-tumour toxicity is not very problematic. However, these are early results in a small number of patients. What is an important finding is that proliferation of CAR-T cells could be traced after the infusion and shows they were not rejected.”
Taking the results of all studies together, Svane reflects on the future of cellular therapies mediated by innovation. “I believe the coming years will see many breakthroughs, although there will be many challenges, including the need to exclude from clinical trials patients who do not express the specific target of interest, or elderly patients who may not be able to receive this type of therapy,” she concludes. “Regulatory issues also represent a key challenge to further progress. The bioinformatic platforms used to generate these novel therapies are rapidly shaping the research landscape, but their assessment and approval still need to be properly defined.”
Abstracts discussed:
Borgers JSW, et al. NTC-001: A phase I study to test safety and efficacy of BNT221, a non-engineered neoantigen-specific T cell product, in patients with advanced or metastatic melanoma. ESMO Congress 2023, Abstract 1017O
Proffered Paper Session – Investigational immunotherapy, 23.10.2023, h. 10:15 – 11:40, Burgos Auditorium – Hall 3
Qi C, et al. Phase I study of GCC CAR-T therapy IM96 in patients with advanced colorectal cancer. ESMO Congress 2023, Abstract 1018O
Proffered Paper Session – Investigational immunotherapy, 23.10.2023, h. 10:15 – 11:40, Burgos Auditorium – Hall 3
Calvo E, et al. Clinical and translational data from the phase 1 SURPASS trial of ADP-A2M4CD8 T-cell receptor (TCR) T-cell therapy alone or combined with nivolumab in solid tumors. ESMO Congress 2023, Abstract 1019O
Proffered Paper Session – Investigational immunotherapy, 23.10.2023, h. 10:15 – 11:40, Burgos Auditorium – Hall 3
Mackensen A, et al. BNT211-01: Interim results from a repeat dose escalation study of CLDN6 CAR-T cells manufactured with an automated process ± a CLDN6-encoding CAR-T cell-Amplifying RNA Vaccine (CARVac). ESMO Congress 2023, LBA35
Mini Oral Session – Developmental therapeutics, 23.10.2023, h. 16:30 – 18:00, Valencia Auditorium – Hall 10