However, presented findings raise some questions about their efficacy in non-injected lesions and the durability of responses
As presented at the ESMO Congress 2024 (Barcelona, 13–17 September), the results of several early-phase studies of investigational oncolytic viruses – a type of immunotherapy by which modified viruses harness the immune system to attack tumours – are showing promise across a range of cancer types, including encouraging responses in a cohort of patients with advanced melanoma who progressed on standard anti-PD-1 therapy.
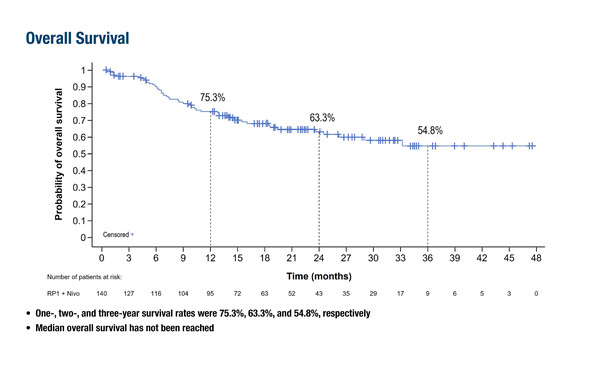
The IGNYTE phase II trial (N=140) investigated the efficacy of RP1, an HSV-1–based oncolytic immunotherapy expressing GM-CSF and a fusogenic protein (GALV-GP-R−), combined with nivolumab in patients with advanced melanoma who had progressed on standard anti-PD-1 therapy; LBA46). In the study, 65.7% of patients had primary resistance to anti-PD-1 treatment, and the response rate was 33.6%. Landmark overall survival (OS) rates at 1 and 2 years were 75.3% (95% confidence interval [CI] 66.9–81.9) and 63.3% (95% CI 53.6–71.5), respectively; median response duration was 21.6 months, median OS was not reached. “The response rate and relatively mild toxicity profile are highly encouraging for this patient population,” comments Dr Marco Donia of the National Center for Cancer Immune Therapy (CCIT-DK), Copenhagen University Hospital Herlev, Denmark. “However, it is difficult to comment on the survival data in the absence of a control group. In addition, patients need injectable lesions and this is reflected by the relatively low proportion of patients having stage IVB–D disease.”
Of the four early-phase trials of oncolytic viral therapy presented at the Congress, two explored combinations of the genetically-engineered adenovirus LOAd703 with either immunotherapy in patients with advanced melanoma or chemotherapy in patients with advanced solid tumours. A combination of LOAd703 with atezolizumab was generally well tolerated and associated with median OS of 19.3 months, with 1- and 2-year survival rates of 58% and 46%, respectively, among 24 patients with stage IV melanoma (Abstract 988O). There was evidence of LOAd703 mediating an inflamed tumour microenvironment with T-cell activation and recruitment. When combined with various chemotherapy regimens, LOAd703 was associated with an overall response rate of 15% in 41 patients with a variety of advanced solid tumours, with disease control for ≥5 months reported in 22% of patients, with evidence of T-cell and DC activation (Abstract 989O). Median OS was longest with a higher dose of LOAd703 (4.4 months at 5x1010 viral particles [VP], 8.4 months at 1x1011 VP and 7.3 months at 5x1011 VP), and the regimen was generally well tolerated. “There is an established rationale for combining an oncolytic virus with immunotherapy, as it is thought this may further potentiate the immune response primarily through the induction of immunogenic cell death. The rationale for combining oncolytic virotherapy with chemotherapy is less established; however, chemotherapy can reduce tumour burden and potentiate immunogenic cell death induced by these viruses,” says Donia.
Two further presentations at the ESMO Congress 2024 report the results of small dose-finding phase I studies with the oncolytic viruses VG2025 (Abstract 994MO) and OVV-01 (Abstract 993MO) as monotherapy in patients with advanced solid tumours. These early-phase studies reported objective responses ranging from 13.0% to 27.3% and treatments were well tolerated. Donia comments, “It is interesting that these studies have enrolled patients with such a diverse range of tumour types that are often resistant to checkpoint inhibitor immunotherapy, including soft tissue sarcoma, and colorectal, liver, ovarian and pancreatic cancers. It is encouraging that antitumour activity has been broadly demonstrated, and while patient numbers are small, these preliminary data highlight the therapeutic potential of oncolytic viruses across cancer types.”
While the latest data are promising, Donia explains that oncolytic viruses are not without limitations: “These treatments have a favourable safety profile, but are primarily injected intratumourally, so there needs to be a lesion to inject. This is relatively straightforward if there is an obvious skin lesion but can be more challenging in the case of less accessible lesions, for example liver metastases, that rely on ultrasound-guided administration.” This also raises the question of the ability of these agents to induce responses in non-injected lesions. “Responses are typically more pronounced in injected lesions, although in the IGNYTE trial, responses were reported in both injected and non-injected lesions, including visceral lesions,” he comments.
Questions also remain as to how the different oncolytic viruses and other intratumourally-injected agents – of which there are now many in development – compare, as there are currently no head-to-head trials. And will these treatments improve survival? “That is a hard endpoint that doctors and patients want to see, in addition to improved patient quality of life. So far, none of the oncolytic viruses has shown an OS benefit in patients with metastatic disease,” replies Donia. As previously reported, while T-VEC is an active oncolytic virus that is EMA-approved and can induce durable responses in patients with advanced melanoma, a phase III trial of T-VEC combined with pembrolizumab failed to improve progression-free survival or OS compared with placebo–pembrolizumab (J Clin Oncol. 2023;41:528–540). “The data from the IGNYTE trial are highly encouraging, and we need to wait to see if these high response rates in patients with anti-PD-1 resistance may translate into prolonged survival,” he concludes.
Programme details
Hamid O, et al. A phase I/II trial (LOKON003) evaluating tumor microenvironment (TME) gene engineering using a viral vector combined with atezolizumab in advanced malignant melanoma. ESMO Congress 2024, Abstract 988O
Proffered Paper Session – Investigational immunotherapy, 13.09.2024, h. 16:00 – 17:30, Burgos Auditorium – Hall 5
Hahn A, et al. Novel gene therapy in advanced solid malignancies: a phase I/II clinical trial. ESMO Congress 2024, Abstract 989O
Proffered Paper Session – Investigational immunotherapy, 13.09.2024, h. 16:00 – 17:30, Burgos Auditorium – Hall 5
Robert C, et al. Primary efficacy, safety, and survival data from the registration-intended cohort of patients with anti–PD-1–failed melanoma from the IGNYTE clinical trial with RP1 combined with nivolumab. ESMO Congress 2024, LBA46
Mini Oral Session – Melanoma and other skin tumours, 15.09.2024, h. 14:45 – 16:15, Oviedo Auditorium – Hall 3
Shen Y, et al. The updated report of phase I trial of VG2025, a non-attenuated HSV-1 oncolytic virus expressing IL-12 and IL-15/Rα payloads, in patients with advanced solid tumors. ESMO Congress 2024, Abstract 994MO
Mini Oral Session – Investigational immunotherapy, 16.09.2024, h. 10:15 – 11:45, Granada Auditorium – Hall 6
Hua, Y, et al. A phase 1, open-label, multicenter, dose escalation safety and tolerability study of oncolytic virus OVV-01 in advanced solid tumors. ESMO Congress 2024, Abstract 993MO
Mini Oral Session – Investigational immunotherapy, 16.09.2024, h. 10:15 – 11:45, Granada Auditorium – Hall 6