Efficacy of PD-1 inhibitors in this setting reflects that reported in triple-negative breast cancer and is confirmed by long-term data presented at the ESMO Congress
Data from two phase III trials showing a benefit of adding neoadjuvant immunotherapy to chemotherapy in hormone receptor (HR)-positive early breast cancer were revealed in presentations at the ESMO Congress 2023 (Madrid, 20–24 October), along with updated data for triple-negative breast cancer (TNBC).
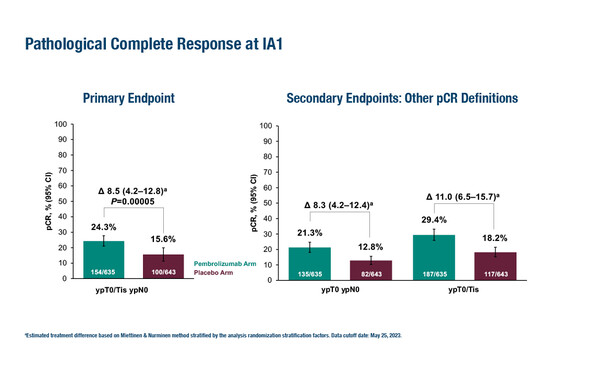
KEYNOTE-756 showed a significantly increased rate of pathological complete response (pCR – a primary endpoint; ypT0/Tis ypN0]) with pembrolizumab plus chemotherapy versus placebo plus chemotherapy (24.3% versus 15.6%; treatment difference 8.5%, 95% confidence interval [CI] 4.2–12.8; p=0.00005) (LBA21). The pCR benefit was generally consistent across pre-specified subgroups including tumour PD-L1 status (CPS ≥1 versus <1), nodal involvement (positive versus negative) and oestrogen receptor (ER) positivity (≥10% versus <10%). Results for the other primary endpoint, event-free survival (EFS), are still immature. In the neoadjuvant phase, grade ≥3 treatment-related adverse event (TRAE) rates were 52.5% with pembrolizumab versus 46.4% with placebo. In the trial, 1,278 patients with grade 3, invasive ductal ER-positive/HER2-negative breast cancer were randomised to neoadjuvant pembrolizumab or placebo plus chemotherapy, then after definitive surgery, patients received pembrolizumab or placebo plus endocrine therapy.
“Evidence that pembrolizumab is effective in HR-positive breast cancers has been lacking so far. However, while insightful, the improvement in pCR is not dramatic and, as these are early data, we do not yet know if this is predictive of enhanced post-surgical EFS, although the pCR endpoint in neoadjuvant therapy is generally a predictor of overall outcome,” cautions Prof. Stephen Johnston from The Royal Marsden NHS Foundation Trust and Institute of Cancer Research, London, UK, commenting on these results. “As expected, pCR rates with chemotherapy are typically very low, representing the heterogeneity of HR-positive breast cancer, which tends to be treated with hormonal and targeted treatments.”
A similar pCR benefit for women with ER-positive/HER2-negative grade 2 or 3 breast cancer was reported in a second trial, the CheckMate 7FL, investigating neoadjuvant nivolumab plus chemotherapy versus placebo plus chemotherapy followed by adjuvant nivolumab or placebo plus endocrine therapy (LBA20). As presented in Madrid, pCR (ypT0/Tis ypN0) rates with neoadjuvant nivolumab plus chemotherapy were significantly improved compared with control (24.5% versus 13.8%; odds ratio [OR] 2.05; 95% CI 1.29–3.27; p=0.0021) and the benefit with nivolumab was greater in PD-L1+ patients (44.3% versus 20.2% with placebo; OR 3.11; 95% CI 1.58–6.11). In the neoadjuvant phase, grade 3–4 TRAE incidence was similar across arms (35% versus 32%). Johnston notes, “Nivolumab is not currently approved for breast cancer. Although promising, the results from CheckMate 7FL show most benefit in PD-L1+ positive patients, which account for only around one-third of the 521 patients involved, so further research is needed to confirm the benefit of this combination therapy before it enters the clinic.”
In TNBC, pembrolizumab is already approved in the neoadjuvant setting based on the positive findings of KEYNOTE-522 (N Engl J Med. 2020;382:810–821). At the ESMO Congress 2023, longer-term updated data were presented from KEYNOTE-522 (LBA18). After a median follow-up of 63.1 months, neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab continued to show improved EFS versus neoadjuvant chemotherapy alone (81.3% versus 72.3%; hazard ratio [HR] 0.63, 95% CI 0.49–0.81) in 1,174 patients with early TNBC, regardless of pCR. Median EFS was not reached in either group. The EFS benefit with pembrolizumab was consistent across pre-specified subgroups (including PD-L1 expression and nodal status). Johnston notes, “This update provides 5-year follow-up data, which is a key landmark for assessing patient outcomes, and shows that the EFS benefit with pembrolizumab is maintained, although OS data are not yet available.”
Negative data for the PD-L1 inhibitor atezolizumab were reported from 280 patients with TNBC in the NeoTRIP study (LBA19). After a median follow-up of 54 months, EFS rates were 70.6% with neoadjuvant atezolizumab plus chemotherapy versus 74.9% with neoadjuvant chemotherapy alone (HR 1.076; 95% CI 0.670–1.731). Johnston advises, “Atezolizumab works differently to pembrolizumab and nivolumab in that it targets PD-L1 on tumour cells rather than PD-1 on T cells, so this may help explain these statistically negative results, although subgroup analyses are ongoing.”
Taken together these findings consolidate the role of immunotherapy in different breast cancer subtypes, with a more positive trend for some agents targeting PD-1 rather than PD-L1. “Data presented in HR-positive breast cancer may be potentially practice-changing,” concludes Johnston. “The next steps will be to determine which patients derive the most benefit, as immunotherapy has side-effects and both studies reported deaths in patients with early-stage disease. Further study is also needed to determine whether immunotherapy is required after surgery if pCR is achieved.”
Abstracts discussed:
Schmid P, et al. Pembrolizumab or placebo plus chemotherapy followed by pembrolizumab or placebo for early-stage TNBC: Updated EFS results from the phase 3 KEYNOTE-522 study. ESMO Congress 2023, LBA18
Proffered Paper Session – Breast cancer, early stage, 20.10.2023, h. 14:00 – 15:40, Bilbao Auditorium – NCC
Gianni L, et al. Event-free survival (EFS) analysis of neoadjuvant taxane/carboplatin with or without atezolizumab followed by an adjuvant anthracycline regimen in high-risk triple negative breast cancer (TNBC): NeoTRIP Michelangelo randomized study. ESMO Congress 2023, LBA19
Proffered Paper Session – Breast cancer, early stage, 20.10.2023, h. 14:00 – 15:40, Bilbao Auditorium – NCC
Loi S, et al. A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) ± NIVO in patients (pts) with high-risk, ER+ HER2− primary breast cancer (BC). ESMO Congress 2023, LBA20
Proffered Paper Session – Breast cancer, early stage, 20.10.2023, h. 14:00 – 15:40, Bilbao Auditorium – NCC
Cardoso F, et al. KEYNOTE-756: Phase 3 study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2– breast cancer. ESMO Congress 2023, LBA21
Proffered Paper Session – Breast cancer, early stage, 20.10.2023, h. 14:00 – 15:40, Bilbao Auditorium – NCC