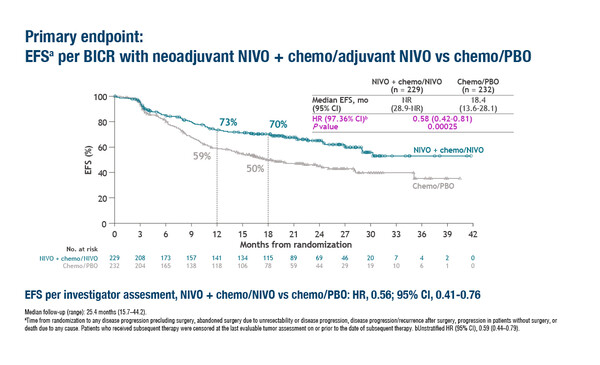
CheckMate 77T demonstrates significantly improved event-free survival with neoadjuvant nivolumab plus chemotherapy followed by adjuvant nivolumab compared with chemotherapy and adjuvant placebo
According to interim results from the phase III CheckMate 77T trial presented in a Presidential Symposium at the ESMO Congress 2023 (Madrid, 20–24 October), neoadjuvant nivolumab plus chemotherapy followed by adjuvant nivolumab significantly improved median event-free survival (EFS) compared with chemotherapy plus adjuvant placebo (not reached versus 18.4 months; hazard ratio 0.58; 97.36% confidence interval [CI] 0.42–0.81; p=0.00025) at a minimum follow-up of 15.7 months (LBA1). The trial involved 461 patients with untreated resectable stage IIA–IIIB non-small-cell lung cancer (NSCLC) of whom 77% underwent definitive surgery.
The benefits of neoadjuvant nivolumab plus chemotherapy/nivolumab over chemotherapy/placebo were also seen in pathological complete response (pCR) rates (25.3% versus 4.7%, respectively; odds ratio [OR] 6.64; 95% CI 3.40–12.97) and major pathological response rates (35.4% versus 12.1%, respectively; OR 4.01; 95% CI 2.48–6.49). Grade 3–4 treatment-related adverse events (AEs) were seen in 32% of patients receiving nivolumab plus chemotherapy/nivolumab and 25% of those receiving chemotherapy/placebo.
“CheckMate 77T shows very promising results to support the use of perioperative nivolumab in resectable NSCLC,” comments Prof. Marina Garassino from the University of Chicago, IL, USA. “In particular, the trial demonstrated that patients could achieve incredible disease control rates. These are very encouraging findings for perioperative immunotherapy.”
Another presentation at the Congress, on the KEYNOTE-671 trial with perioperative pembrolizumab, demonstrated an advantage not only for prolonged EFS, but also for overall survival for the first time (LBA56). In this trial, patients with resectable stage II, IIIA and IIIB (T3-4N2) NSCLC received pembrolizumab or placebo plus cisplatin-based chemotherapy followed by surgery and then adjuvant pembrolizumab or placebo. Previously, the CheckMate 816 trial has shown significantly longer EFS and a higher percentage of patients with a pCR when they received neoadjuvant nivolumab plus chemotherapy versus chemotherapy before surgery and then no subsequent treatment (N Engl J Med. 2022;386:1973–1985). Garassino thinks the focus of investigation should now shift towards evaluating the benefit of the adjuvant part of the regimen, assessing which patients benefit from this component. “I believe the academic community should run a new trial comparing adjuvant versus non-adjuvant treatment in patients who previously received neoadjuvant treatment,” she says, then concludes, “For the time being, neoadjuvant treatment should be considered in all patients with a resectable tumour and I think the adjuvant part should be discussed on an individual patient-by-patient basis.”
Abstracts discussed:
Spicer JD, et al. Overall survival in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage non-small-cell lung cancer (NSCLC). ESMO Congress 2023, LBA56
Proffered Paper Session – Non-metastatic NSCLC and other thoracic malignancies, 20.10.2023, h. 14:00 – 15:45, Sevilla Auditorium – Hall 9
Cascone T, et al. CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. ESMO Congress 2023, LBA1
Presidential 1, 21.10.2023, h. 16:30 – 18:15, Madrid Auditorium – Hall 6