Negative trials presented at the ESMO Congress 2023 suggest that a new treatment strategy may be required for patients with metastatic disease
Although checkpoint inhibitors have shown remarkable efficacy in many solid tumours, their performance in prostate cancer has been disappointing so far. Some preliminary evidence has suggested a potential synergy between androgen receptor pathway inhibitors (ARPIs) and PD-1-targeted therapies (J Clin Oncol. 2018;36_suppl 15:5047; Ann Oncol. 2020;31_suppl 4:625P). However, the latest findings from several clinical trials presented at the ESMO Congress 2023 (Madrid, 20–24 October) cast doubt on the efficacy of this combination of therapies in both metastatic castration-resistant prostate cancer (mCRPC) and metastatic hormone-sensitive prostate cancer (mHSPC).
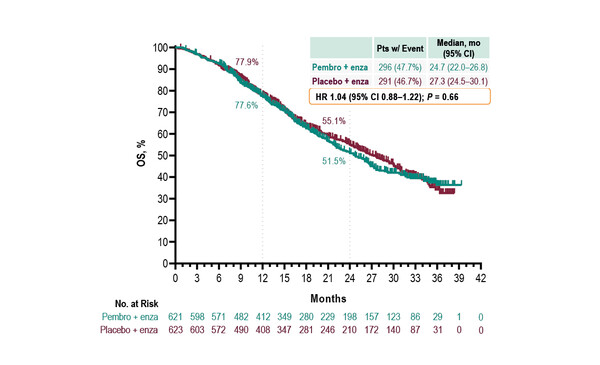
As presented in a Mini Oral Session, the phase III KEYNOTE-641 study, which investigated the combination of pembrolizumab and enzalutamide versus placebo plus enzalutamide in 1,244 patients with mCRPC failed to meet its dual primary endpoints of radiographic progression-free survival (rPFS) and overall survival (OS) (Abstract 1771MO). The modest increase in complete response rate with pembrolizumab plus enzalutamide versus placebo plus enzalutamide (7.4% versus 2.7%, respectively) did not translate into significant increases in rPFS (median 10.4 months versus 9.0 months, respectively; hazard ratio [HR] 0.98, 95% confidence interval [CI] 0.84−1.14) or OS (median 24.7 months versus 27.3 months, respectively; HR 1.04, 95% CI 0.88−1.22). Moreover, there was an increase in the incidence of treatment-related adverse events (TRAEs) with the addition of pembrolizumab compared with placebo plus enzalutamide. Dr Himisha Beltran from the Dana-Farber Cancer Institute, Harvard Medical School, Boston, USA, notes: “Although the results from this trial were negative, hopefully there are still lessons we can learn. We know that a small number of patients respond very well to immunotherapy and some of these harbour tumour mismatch repair deficiency or microsatellite instability. Beyond these patients, the challenge now will be to understand if there are other novel combinations that can sensitise to immunotherapy and identify the patients who may benefit from these treatments.”
In addition, the results of the combination of pembrolizumab plus enzalutamide in 1,251 patients with mHSPC in the phase III KEYNOTE-991 study were presented, with the trial failing to reach its primary endpoint of rPFS (HR 1.20, 95% CI 0.96–1.49) and forcing its early termination for futility (Abstract 1772MO). An increase in TRAEs with pembrolizumab was again observed. Similar results were reported in a poster presentation of the pilot phase of the PROSTRATEGY study of ipilimumab and/or nivolumab combined with ADT plus docetaxel in 150 patients with high-volume mHSPC (Abstract 1783P). After a median follow-up of 32.5 months, no significant differences in efficacy outcomes, including rPFS, clinical PFS and OS, were detected between treatment arms. Commenting on these findings, Beltran says, “We had hoped to obtain more positive results in mHSPC. These trials were based on the scientific premise that primary ADT increases immune infiltration in tumours, which can be further enhanced by immune modulators, such as enzalutamide or docetaxel, potentially enhancing response to immunotherapy. However, the results for both of these studies were negative. Overall, these studies are adding to an overall landscape of negative trials for immunotherapy in prostate cancer. Other trials are ongoing, but as a field, we may need to rethink our strategy for these patients.”
Regarding the possible reasons why patients with prostate cancer are not responding to immunotherapy in these trials, Beltran suggests: “It may be that only a fraction of patients are responding to treatment or perhaps the immune effects elucidated from the combination are too transient or that immunosuppressive cells still predominate. Looking ahead, it is essential that we work to better understand mechanisms of resistance to immunotherapy, find ways to improve patient selection and also that we consider different combinations of therapies, including those that target novel pathways.”
Abstracts discussed:
Graff JN, et al. Pembrolizumab (pembro) plus enzalutamide (enza) for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): randomized double-blind phase 3 KEYNOTE-641 study. ESMO Congress 2023, Abstract 1771MO
Mini Oral Session – Genitourinary tumours, prostate, 22.10.2023, h. 08.30 – 09.35, Granada Auditorium – Hall 3
Gratzke CJ, et al. Pembrolizumab (pembro) plus enzalutamide (enza) and androgen deprivation therapy (ADT) for patients (pts) with metastatic hormone-sensitive prostate cancer (mHSPC): randomized double-blind phase 3 KEYNOTE-991 study. ESMO Congress 2023, Abstract 1772MO
Mini Oral Session – Genitourinary tumours, prostate, 22.10.2023, h. 08.30 – 09.35, Granada Auditorium – Hall 3
Arranz Arija JA, et al. PROSTRATEGY: A SOGUG randomized trial of androgen deprivation therapy (ADT) plus docetaxel (dct) +/- nivolumab (nivo) or ipilimumab-nivolumab (ipi-nivo) in high-volume metastatic hormone-sensitive prostate cancer (hvHSPCa). Efficacy results from the pilot phase. ESMO Congress 2023, Abstract 1783P
Poster Display Session – Prostate cancer, 22.10.2023, h. 12.00 – 13.00, Hall 8