Positive outcomes in advanced or metastatic disease reported from EV-302/KEYNOTE-A39 and CheckMate 901 indicate an alternative to first-line chemotherapy for the first time
Despite chemotherapy and immunotherapy, long-term survival remains poor for patients with advanced bladder cancer and there is an urgent need for innovative treatment combinations to improve survival. In a Presidential Symposium at the ESMO Congress 2023 (Madrid, 20–24 October), practice-changing results were presented from two phase III trials in patients with previously untreated, locally advanced or unresectable metastatic urothelial carcinoma.
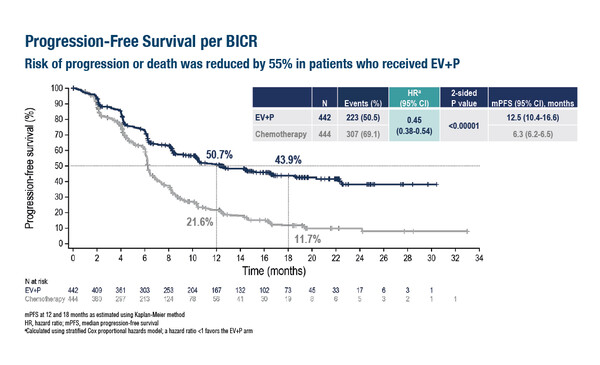
In the EV-302/KEYNOTE-A39 trial, a combination of the nectin 4-directed antibody–drug conjugate (ADC) enfortumab vedotin with pembrolizumab almost doubled median progression-free survival (PFS) (12.5 months versus 6.3 months, respectively; hazard ratio [HR] 0.45; 95% confidence interval [CI] 0.38–0.54; p<0.00001) and median overall survival (OS) (31.5 months versus 16.1 months, respectively; HR 0.47; 95% CI 0.38–0.58; p<0.00001) compared with chemotherapy (cisplatin or carboplatin plus gemcitabine) at a median follow-up of 17.2 months in 886 patients with previously untreated, locally advanced or metastatic urothelial carcinoma (LBA6).
Enfortumab vedotin plus pembrolizumab also led to a significant increase in the overall response rate compared with chemotherapy (67.7% versus 44.4%, respectively; p<0.00001). The most common grade ≥3 treatment-related adverse events (TRAEs) of special interest for enfortumab vedotin included skin reactions (15.5%), peripheral neuropathy (6.8%) and hyperglycaemia (6.1%). Commenting on these findings, Dr Andrea Apolo from the National Institutes of Health, Bethesda, MD, USA, says, “The results of this study have been long awaited as for more than two decades, platinum-based chemotherapy has been the standard of care and it is very exciting that we have now identified a combination of treatments that is superior to chemotherapy in terms of OS.”
The CheckMate 901 study is the first phase III trial showing a beneficial impact of an upfront combination of chemotherapy plus immunotherapy. Adding nivolumab to chemotherapy (gemcitabine–cisplatin) significantly prolonged median OS (21.7 months versus 18.9 months; HR 0.78; 95% CI 0.63–0.96; p=0.0171) and median PFS (7.9 months versus 7.6 months; HR 0.72; 95% CI 0.59–0.88; p=0.0012) compared with chemotherapy alone at a median follow-up of 33.6 months in 608 patients with previously untreated, unresectable or metastatic urothelial carcinoma (LBA7). Objective response rates were 57.6% with nivolumab plus chemotherapy and 43.1% with chemotherapy alone, with corresponding complete response rates of 21.7% and 11.8%. Grade ≥3 TRAEs occurred in 62% of patients receiving nivolumab plus chemotherapy and 52% receiving chemotherapy. “This is the first study to show an improvement in OS using a combination of chemotherapy and immunotherapy in patients with metastatic bladder cancer,” notes Apolo. “This is highly encouraging given that previous phase III studies of checkpoint immunotherapy in this disease (atezolizumab in IMvigor130 and pembrolizumab in KEYNOTE-361) failed to show a statistical improvement in OS. It is interesting to note that patients in CheckMate 901 all received cisplatin whereas patients in IMvigor130 and KEYNOTE-361 received either carboplatin or cisplatin. It may therefore be that advanced bladder cancer is particularly sensitive to the specific combination of nivolumab and cisplatin.”
The two studies presented are highly significant for patients as they are the first trials to challenge the longstanding standard of first-line platinum-based chemotherapy for patients with advanced/metastatic urothelial carcinoma. “The data for enfortumab vedotin and pembrolizumab are particularly impressive and this combination will clearly become the new standard of cancer care for this cohort of patients,” concludes Apolo. “The field of urothelial carcinoma is evolving and it is fantastic news that we can now improve survival for our patients. Multiple clinical trials are ongoing, such as the phase III NILE study that is investigating durvalumab plus chemotherapy versus durvalumab with tremelimumab plus chemotherapy versus chemotherapy alone (NCT03682068), and we are eagerly awaiting their results.”
Abstracts discussed:
Powles TB, et al. EV-302/KEYNOTE-A39: Open-label, randomized phase 3 study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). ESMO Congress 2023, LBA6
Presidential Symposium 2, 22.10.2023, h. 16:30 – 18:15, Madrid Auditorium – Hall 6
Van der Heijden MS, et al. Nivolumab plus gemcitabine-cisplatin versus gemcitabine-cisplatin alone for previously untreated unresectable or metastatic urothelial carcinoma: results from the phase 3 CheckMate 901 trial. ESMO Congress 2023, LBA7
Presidential Symposium 2, 22.10.2023, h. 16:30 – 18:15, Madrid Auditorium – Hall 6