Long-term data from monarchE supports dose reduction while NATALEE data reinforces consistency across subgroups
Emerging data support the use of cyclin-dependent kinase (CDK) inhibitors as a treatment for hormone receptor (HR)-positive/HER2-negative early breast cancer, with promising results reported recently from the phase III monarchE (Lancet Oncology. 2023;24:77–90) and NATALEE studies (J Clin Oncol. 2023;41_suppl 17:LBA500) of abemaciclib and ribociclib, respectively. Additional insights from these trials were presented at the ESMO Congress 2023 (Madrid, 20–24 October), confirming long-term efficacy and supporting dose reduction as well as confirming consistent efficacy across subgroups.
Five-year efficacy data after a median follow-up of 54 months showed that patients treated with abemaciclib plus endocrine therapy (ET) in the monarchE trial had a reduced risk of disease recurrence compared with patients treated with ET only, with a hazard ratio of 0.680 (95% confidence interval [CI] 0.599–0.772) for invasive disease-free survival (IDFS) and 0.675 (95% CI 0.588–0.774) for distant relapse-free survival (DRFS) (LBA17). There were numerically fewer deaths in the abemaciclib arm compared to the ET-only arm (208 versus 234, respectively) and no new safety signals were observed. Commenting on these findings, Dr Carmen Criscitiello from the European Institute of Oncology, Milan, Italy, states, “It is very important that we are seeing a sustained benefit with abemaciclib beyond completion of the treatment. The Kaplan-Meier curves have continued to separate over time and show an absolute five-year improvement in IDFS and DRFS of 7.6% and 6.7%, respectively. The long-term safety profile is also very reassuring.”
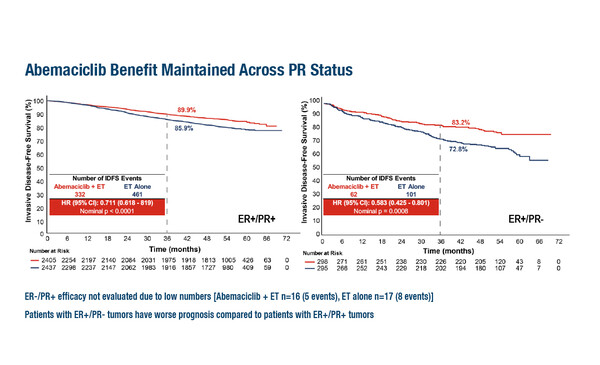
Further data from the monarchE trial showed that the treatment effect of abemaciclib on IDFS at 54 months of follow-up was consistent irrespective of Ki-67, oestrogen receptor (ER) and progesterone receptor (PR) expression as prognostic and predictive biomarkers (Abstract 240MO). Patients with ER+/PR– tumours had a worse prognosis than those with ER+/PR+ tumours. IDFS rates at 36 months in patients treated with abemaciclib plus ET were 89.9% for those with ER+/PR+ tumours and 83.2% for those with ER+/PR– tumours. For patients treated with ET only, IDFS rates were 85.9% for ER+/PR+ tumours and 72.8% for ER+/PR– tumours. A significant treatment benefit was observed with abemaciclib in both ER+/PR+ and ER+/PR– subgroups. Criscitello notes, “These data further contribute to our confidence in these agents as they demonstrate that patients can benefit regardless of Ki-67, ER or PR expression. While we know that ER+/PR– tumours are less likely to respond to ET, these data reveal that this is not the case for patients treated with the combination of ET and abemaciclib.”
Almost half of the patients in the monarchE trial required dose reductions of abemaciclib to manage adverse events. The results of an exploratory analysis presented at the Congress revealed that among the 2,791 patients treated with abemaciclib, 832 (30%) and 389 (14%) required one or two dose reductions, respectively (Abstract 274P). When the impact of these dose reductions on efficacy was examined in subgroups defined by relative dose intensity, 4-year IDFS rates ranged from 83.7% to 87.1%. Using a time-dependent Cox proportional hazards model, the effect of abemaciclib appeared similar when administered at a twice-daily dose of 150 mg versus either 100 mg or 50 mg (hazard ratio 0.905, 95% CI 0.727–1.125). Criscitello notes, “This outcome is not unexpected as we know that dose reduction does not compromise efficacy in the metastatic setting. However, it is very important in the curative setting for the agent to be well-tolerated so that patients can continue to receive treatment for an extended period whilst maintaining a good quality of life.”
Finally, the results of a pre-specified exploratory subgroup analysis from the NATALEE trial presented at the Congress revealed that the 3-year IDFS benefit of ribociclib combined with a non-steroidal aromatase inhibitor (NSAI) versus an NSAI alone was consistent across all clinically relevant subgroups (LBA23). The analysis included 5,101 patients with a median follow-up of 27.7 months and examined subgroups, such as menopausal status, anatomical stage, nodal status, age and Ki-67 index. “These findings align with the overall results from the NATALEE trial and support the use of ribociclib as standard of care for a broad population of patients with HR-positive+/HER2-negative early breast cancer,” says Criscitiello.
Summarising all the findings from the monarchE and NATALEE studies, Criscitiello concludes: “The fact that additional analyses from both trials confirm previous results is very reassuring since we have already implemented this treatment regimen into clinic practice. Also, it is very important to have confirmation that a dose reduction for CDK inhibitors is feasible, thus enabling us to offer our patients more tolerable treatment options and improved quality of life.”
Abstracts discussed:
Harbeck N, et al. Adjuvant abemaciclib plus endocrine therapy for HR+, HER2-, high-risk early breast cancer: results from a preplanned monarchE overall survival interim analysis, including 5-year efficacy outcomes. ESMO Congress 2023, LBA17
Proffered Paper Session – Breast cancer, early stage, 20.10.2023, h. 14.00 – 15.40, Bilbao Auditorium – NCC
O’Shaughnessy J, et al. Impact of dose reductions on efficacy of adjuvant abemaciclib for patients with high risk early breast cancer (EBC): Analyses from the monarchE study. ESMO Congress 2023, Abstract 274P
Poster Display Session – Breast cancer, early stage, 21.10.2023, h. 12.00 – 13.00, Hall 8
Goetz MP, et al. Prognostic and predictive impact of estrogen/progesterone receptor (ER/PR), and Ki-67 expression: an exploratory analysis from the monarchE trial in patients with high-risk, HR+, HER2-, early breast cancer (EBC). ESMO Congress 2023, Abstract 240MO
Mini Oral Session – Breast cancer, early stage, 23.10.2023, h. 10.15 – 11.45, Bilbao Auditorium – NCC
Bardia A, et al. Invasive disease–free survival (iDFS) across key subgroups from the Phase III NATALEE study of ribociclib (RIB) + a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+/HER2− early breast cancer (EBC). ESMO Congress 2023, LBA23
Mini Oral Session – Breast cancer, early stage, 23.10.2023, h. 10.15– 11.45, Bilbao Auditorium – NCC