Promising results reported for datopotamab deruxtecan and trastuzumab deruxtecan in HER2-non-amplified breast cancer
Antibody–drug conjugates (ADCs) are rapidly changing the treatment landscape for inoperable or metastatic breast cancer. At the ESMO Congress 2023 (Madrid, 20–24 October), favourable results with two ADCs, datopotamab deruxtecan and trastuzumab deruxtecan, were reported from large, randomised trials.
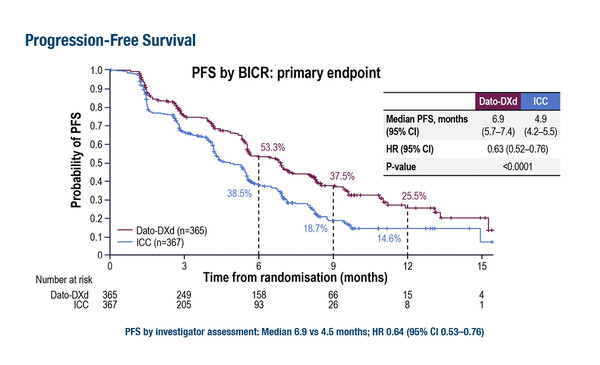
The TROPION-Breast01 trial met its primary endpoint, with significantly improved progression-free survival (PFS) reported with the TROP2-directed ADC, datopotamab deruxtecan versus investigator’s choice of chemotherapy (6.9 months versus 4.9 months; hazard ratio [HR] 0.63, 95% confidence interval [CI] 0.52–0.76; p<0.0001) in 732 patients with inoperable or metastatic hormone receptor-positive, HER2-negative breast cancer who had received previous chemotherapy (LBA11). The PFS benefit was consistent across the subgroups studied. Overall survival (OS) rates favoured the datopotamab deruxtecan group (HR 0.84, 95% CI 0.62–1.14), although the data are not yet mature.
“These positive results are expanding the list of ADCs that have proven to be more effective than conventional chemotherapy in metastatic breast cancer lacking HER2 amplification,” says Dr Sarat Chandarlapaty from the Memorial Sloan Kettering Cancer Center, New York, NY, USA. Similar results have been reported for this population with trastuzumab deruxtecan in the DESTINY-Breast04 trial (N Engl J Med. 2022;387:9–20) and with sacituzumab govitecan in the TROPiCS-02 trial (J Clin Oncol. 2022;40:3365–3376). “However,” he continues, “we have limited data to guide which of these three ADCs should be administered to any given patient and whether any are effective after progression on one of the others.” According to Chandarlapaty, results on the safety profile of datopotamab deruxtecan are reassuring, with only 2/360 patients experiencing grade ≥3 drug-related interstitial lung disease (ILD) and the overall lower rate (21%) of grade ≥3 treatment-related adverse events observed compared with chemotherapy (45%).
Also presented in Madrid were updated survival data from the DESTINY-Breast04 trial and these indicate a sustained improvement in OS (Abstract 376O). After a median follow-up of 32.0 months, median OS was 22.9 months for the 373 patients treated with trastuzumab deruxtecan and 16.8 months for the 184 patients treated with treatment of physician’s choice (TPC; HR 0.69, 95% CI 0.55–0.86). Median PFS was 8.8 months for the trastuzumab deruxtecan arm and 4.2 months for the TPC arm (HR 0.36, 95% CI 0.29–0.45). There was also a 42% reduction in the risk of death (HR 0.58, 95% CI 0.31–1.08) and a 71% reduction in the risk of disease progression or death (HR 0.29, 95% CI 0.15–0.57) with trastuzumab deruxtecan versus TPC in the cohort of patient with a hormone receptor-negative tumour. Commenting on these findings, Chandarlapaty notes, “These results are highly concordant and encouragingly confirmatory of the primary results on the efficacy of trastuzumab deruxtecan in HER2-low advanced breast cancer and there were no new safety signals identified with longer follow-up. The overall rate of grade 5 treatment-related ILD rose slightly from 0.8% to 1.1%, and although rare, ILD remains an important consideration requiring ongoing clinical vigilance.”
Chandarlapaty puts these results into context and looks to the future, saying, “Consistent benefit has been observed in breast cancer with ADCs bearing payloads that target topoisomerase I, whether the antigen target has been HER2, TROP2 or HER3. We now need further pharmacological strategies to improve the duration of response in HER2-low breast cancer to a similar extent as seen in HER2-overexpressing breast cancer. Emerging questions in the field also relate to mechanisms of resistance and how these alter sensitivity to other ADCs within the class.”
Abstracts discussed:
Modi S, et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Updated survival results of the randomized, phase III DESTINY-Breast04 study. ESMO Congress 2023, Abstract 376O
Proffered Paper Session – Breast cancer, metastatic, 21.10.2023, h. 10:15 – 11:35, Barcelona Auditorium – Hall 9
Bardia A, et al. Datopotamab deruxtecan (Dato-DXd) vs chemotherapy in previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2–) breast cancer (BC): Primary results from the randomised phase III TROPION-Breast01 trial. ESMO Congress 2023, Abstract LBA11
Presidential Symposium 3, 23.10.2023, h. 16:30 – 18:15, Madrid Auditorium – Hall 6