Survival benefit from the combination of gemcitabine with paclitaxel and the role of patients’ stratification for FOLFORINOX are investigated in metastatic and locally advanced settings
Data from two phase III trials presented at ESMO Congress 2022 give new information on the management of pancreatic cancer, which has shown little or no improvement in overall survival (OS) in the past decade as well as having very limited treatment options.
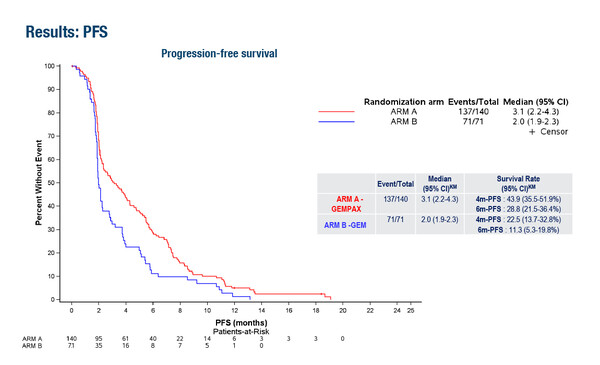
In the PRODIGE 65 – UCGI – 36 – GEMPAX UNICANCER study, patients with metastatic pancreatic cancer were randomised to GEMPAX (gemcitabine 1,000 mg/m² + paclitaxel 80 mg/m² IV infusion on days 1, 8 and 15 every 28 days) or gemcitabine after failure or intolerance to first-line FOLFIRINOX. The combination of gemcitabine with paclitaxel provided no benefit in OS (primary endpoint) but significantly improved both progression-free survival (PFS) and objective response rate (ORR) (LBA60). Median PFS in the GEMPAX arm was 3.1 months (95% confidence interval [CI] 2.2–4.3) versus 2.0 months (95% CI 1.9–2.3) in the gemcitabine arm (hazard ratio [HR] 0.64; 95% CI 0.47–0.89), and the corresponding ORR was 19.2% (95% CI 12.7–27.2) for GEMPAX versus 4.8% (95% CI 1.0–13.3%) for gemcitabine.
“This is the first prospective, randomised, phase III study to test the strategy of giving gemcitabine-based chemotherapy after failure of first-line FOLFIRINOX,” says Dr Teresa Macarulla, Head of the GI Cancer Program Unit at the Institute of Oncology, Barcelona, Spain.
“We already had prospective, randomised, phase III study data to support the use of 5-FU-based chemotherapy after failure of first-line gemcitabine therapy, but not the other way around.” Although the combination of gemcitabine with paclitaxel provided no OS benefit over gemcitabine monotherapy, Macarulla thinks the benefits to secondary outcomes of PFS and ORR could support using this combination if nab-paclitaxel is not available. “There could be a case for giving gemcitabine with paclitaxel in countries where oncologists do not have access to nab-paclitaxel,” she explains, adding: “I would also like to see the study repeated using nab-paclitaxel – which we know works in these patients.”
Also presented at ESMO Congress 2022, the PRODIGE 29-UCGI 26 (NEOPAN) study compared treatment with FOLFIRINOX (every 14 days for 12 cycles) to that with gemcitabine (1,000 mg/m2 on days 1, 8 and 15 for six 28-day cycles except for cycle 1 with an infusion on day 22) in the locally advanced unresectable pancreatic cancer setting (Abstract 1296MO). FOLFIRINOX delivered significantly longer PFS (the primary endpoint) than gemcitabine. Macarulla remarks that: “The most important point about this study is that it is the first to give us prospective, randomised phase III data comparing FOLFIRINOX with the standard of care. We had corresponding data from the metastatic and adjuvant disease settings but until now, not in the locally advanced disease setting.” When asked what impact these data might have on clinical practice, she explains: “We have already been using FOLFIRINOX in these patients based on extrapolating data from the metastatic disease setting. This study just confirms that strategy and gives us some reassurance that using FOLFIRINOX is better than using gemcitabine in the locally advanced setting also.”
Looking to the future for this underserved type of cancer, Macarulla finishes by commenting: “We need biomarkers so we can match the best treatment to each patient instead of treating them all the same way, and we also need more treatment options than just FOLFIRINOX and gemcitabine with nab-paclitaxel. Pancreatic cancer is a very complicated disease with a very complicated biology, and we must continue to do research to find more options for these patients.”
Abstracts presented:
de la Fouchardiere C, et al. Evaluation of gemcitabine and paclitaxel versus gemcitabine alone after FOLFIRINOX failure or intolerance in metastatic pancreatic ductal adenocarcinoma: Results of the randomized phase III PRODIGE 65 – UCGI 36 – GEMPAX UNICANCER study. ESMO Congress 2022, LBA60
Proffered Paper Session 2 – GI, Upper digestive, 11.09.2022, h. 14:45 – 16:15, Brest Auditorium
Ducreux MP, et al. PRODIGE 29-UCGU 26 (NEOPAN): A phase III randomised trial comparing chemotherapy with FOLFIRINOX or gemcitabine in locally advanced pancreatic carcinoma (LAPC). ESMO Congress 2022, Abstract 1296MO
Mini Oral Session – GI, Upper digestive, 10.09.2022, h. 14:45 – 16:15, Toulouse Auditorium