In a head-to-head comparison to the standard of care, tumour-infiltrating lymphocytes show clinical efficacy even in patients who are refractory to anti-PD-1 treatment
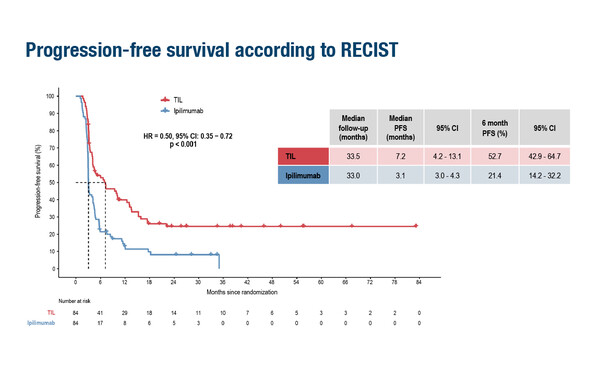
A Presidential Symposium presentation at ESMO Congress 2022 provides results of tremendous clinical significance for tumour-infiltrating lymphocytes (TILs) in advanced melanoma, suggesting a potential new standard of care in this setting. In a randomised, multicentre, open-label, phase III trial, TILs more than halved the risk of progression compared with ipilimumab (3 mg/kg q3 weeks for up to 4 doses), with median progression-free survival (PFS) times of 7.2 months versus 3.1 months, respectively, (hazard ratio 0.50; 95% confidence interval [CI] 0.35–0.72; p<0.001) at a median follow-up of 33.5 months for TILs (n=84) and 33.0 months for ipilimumab (n=84) patients with unresectable stage IIIc–IV melanoma (LBA3).
Most of the patients (86%) were refractory to anti-PD-1 treatment. The overall response rates (ORRs) with TILs and ipilimumab were 48.8% and 21.4%, respectively, with corresponding complete response (CR) rates of 20.2% and 7.1%. Numerically, median overall survival time was longer with TILs (25.8 months versus 18.9 months; HR 0.83; 95% CI 0.54–1.27), but did not reach statistical significance (p=0.39). In the study, patients were stratified for BRAFV600 mutation status, treatment line (first or second) and centre; the primary endpoint was PFS.
Prof. George Coukos of the Ludwig Institute for Cancer Research at the University of Lausanne and University Hospital of Lausanne Centre Hospitalier Universitaire Vaudois, Switzerland explains that these are the very first results from a randomised comparison between TILs and immunotherapy in any tumour type. “TIL adoptive cell therapy (ACT) has been around for almost 20 years, with multiple reports from individual centres of response rates in melanoma ranging from the low percentages to up to 50%. However, until now, no study has attempted randomisation against a standard of care.” He notes, however, that a combination of CTLA-4 and PD-1 blockade, and not ipilimumab, is now considered the standard of care in this setting.
Also, most previous studies of TILs have been conducted in patients who were immunotherapy-naïve. “In the study the vast majority of patients had disease that failed to respond to anti-PD-1 therapy, confirming that a high rate of response can be achieved in patients with anti-PD-1 refractory disease,” says Coukos. With a TILs-associated ORR more than twice that achieved with ipilimumab and a CR rate almost three-times as high, he thinks that the implications of the results for the medical community and for patients are clear, particularly as CRs after TIL ACT have been consistently associated with durable long responses and potential cure. “Melanoma remains an area of unmet medical need,” he says. “In patients with BRAF mutations, responses to kinase inhibitors are often spectacular but are generally not durable, and for patients without BRAF mutations, checkpoint blockade is currently the only available effective therapy. The multiple kinase inhibitor lenvatinib in combination with checkpoint blockade is emerging as a therapeutic possibility. Now we have this study, indicating that a significant proportion of patients with melanoma could not only be helped but potentially cured with TILs.”
An important next step in the study of TILs is identifying the right patients to include in future clinical trials. Finding the patients who are likely to achieve the most benefit is particularly important in view of the toxicity associated with the high-dose chemotherapy lymphodepleting regimen and the use of interleukin-2 regimen to support TILs therapy, which currently require hospitalisation. Related to this, identifying ways to reduce the toxicity of preparative/supportive regimens to enable outpatient treatment is a major aim.
As for the future application of TILs, their use in other solid tumour types looks promising. “Preliminary data already exist from other groups that this approach can potentially be effective in patients with tumours induced by human papilloma virus, such as cervical cancer or head and neck tumours, as well as in non-small-cell lung cancer. The ideal would be to extend this application to all solid tumours,” says Coukos. A key area of investigation should be applying TILs therapy early in the management of cancers, at a time when the immune system has not been largely exhausted by multiple lines of prior therapies and in situations where minimal residual disease can be identified based on current therapy regimens.
Turning away from the results, Coukos also draws attention to the achievement of completing the study presented at ESMO Congress 2022. The complexity of generating and administering TILs means that this technique is currently limited to a few specialised centres throughout the world. “The findings demonstrate the ability of academic institutions to conduct complex studies on a multicentre basis, harmonising manufacturing logistics, the management of patients and data sharing,” he concludes.
Abstract presented:
Haanen J, et al. Treatment with tumor-infiltrating lymphocytes (TIL) versus ipilimumab for advanced melanoma: results from a multicenter, randomized phase 3 trial. ESMO Congress 2022, LBA3
Presidential Symposium I, 10.09.2022, h. 16:30 – 18:00, Paris Auditorium