Recent evidence shows the potential of immunotherapy to change the treatment landscape of triple-negative breast cancer (TNBC), where historically, chemotherapy has been the only systemic option. But, how do we choose what therapy to give which patients?
With the phase III KEYNOTE-522 trial, we have seen practice-changing results, establishing neoadjuvant immunotherapy plus chemotherapy as the standard of care for many patients with this tumour subtype (N Engl J Med. 2020;382:810–821; N Engl J Med. 2022;386:556–567). Since then, several randomised trials have evaluated the effectiveness of adding immunotherapy to chemotherapy in the neoadjuvant setting, with mixed results (NPJ Breast Cancer. 2022;8:23).
We are now at the point where critical questions need to be answered. Firstly, which patients require a checkpoint inhibitor? Not all patients are sufficiently fit for immunotherapy when given along with an aggressive chemotherapy backbone, so the choice of treatment needs to be counterbalanced against quality of life (QoL).
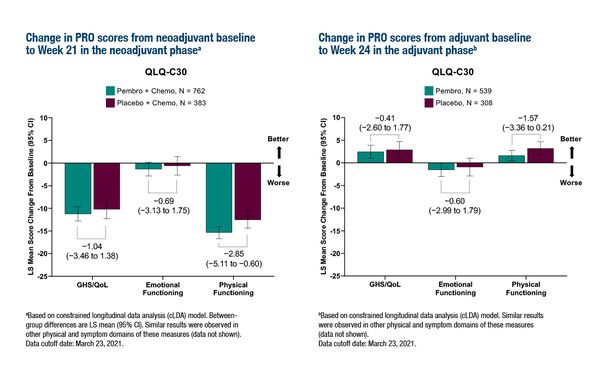
At ESMO Congress 2022, results for the secondary endpoints assessing patient-reported outcomes (PROs) in KEYNOTE-522 – measured using EORTC QLQ-30 and QLQ-BR23 questionnaires during the neoadjuvant and adjuvant stages – were presented (Abstract 135MO). Completion and compliance rates for QLQ-30 were ≥80% at week 21 of the neoadjuvant phase and week 24 of the adjuvant phase. The authors conclude that there were no meaningful differences between treatment groups in PRO scores, including global health status, QoL, emotional functioning, physical functioning and breast symptoms.
In interpreting the PRO data from this trial, it is important to recognise that KEYNOTE-522 is a proof-of-principle trial. Pembrolizumab in addition to an aggressive chemotherapy backbone increases pathological complete response (pCR) rates and improves survival in comparison to the same cytotoxic therapy alone. However, in the real world there are going to be fewer patients than in a clinical trial setting who are fit enough to tolerate this regimen, as they will be older and/or have comorbidities prohibiting them from receiving this treatment.
The PRO data are encouraging and reassure us that there is no general detriment to QoL with the addition of pembrolizumab to chemotherapy. However, the premise of using scores from questionnaires is to provide a broad and general assessment of changes in health-related QoL in the trial, so these data may appear discordant with individual toxicity and individual issues arising from immunotherapy. It is also important to acknowledge that more than 90% of all side effects are due to chemotherapy given in both treatment arms, therefore it is important to ask if the impact of these dominant side effects can be distinguished from immune-related adverse events derived from pembrolizumab.
Finally, we need to bear in mind that patients who benefitted from treatment in the neoadjuvant stage without immune-related adverse events and who continued to the adjuvant stage of the trial will have completed PRO questionnaires, whereas those who experienced immune-related adverse events leading to treatment discontinuation may not have been included in the PRO assessments. It is therefore important to raise awareness of rare but severe toxicities – not all of which are reversible – and counsel patients accordingly.
How can we improve patient selection for optimal treatment of early TNBC? The KEYNOTE-522 trial evaluated pembrolizumab in addition to an aggressive chemotherapy backbone: it is a proof of principle that immunotherapy in addition to maximum chemotherapy increases pCR rates and improves survival. In the real world not all of these patients will need this type of aggressive therapy and so there may be an opportunity to de-escalate chemotherapy and reduce toxicity.
We need more research to identify biomarkers that are predictive of response. To date, we have no validation that biomarkers show a clear interaction with the addition of immunotherapy to chemotherapy. We remain unsure who needs what type of chemotherapy and who truly benefits from the addition of immunotherapy. In the absence of truly predictive biomarkers, the practical solution is to use response in the neoadjuvant setting. Early response by MRI assessment or the evaluation of residual tumour in the surgical specimen are important predictors of survival in TNBC. Perhaps, for the time being, these simple clinical evaluations will spare patients from side effects and protect against financial toxicity.
Abstracts presented:
Dent RA, et al. HRQoL with neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy, followed by adjuvant pembrolizumab vs placebo for early-stage TNBC: results from KEYNOTE-522. ESMO Congress 2022, Abstract 135MO
Mini Oral Session – Breast cancer, early stage, 12.09.2022, h. 08:30 – 10:00, Cannes Auditorium
The three “Ws” of early breast cancer management. ESMO Congress 2022
Educational Session, 10.09.2022, h. 16:30 – 18:00, Cannes Auditorium