In a phase I/II study, the combination treatment was tolerable in men who had progressed on one novel anti-androgen therapy
Phase I/II data presented at ESMO Congress 2022 showed that direct targeting of myeloid chemotaxis has potential as a therapeutic strategy in metastatic castration-resistant prostate cancer (mCRPC) (Abstract 454O).
Results from dose-limiting toxicity (DLT)-evaluable patients with mCRPC who had progressed on one novel anti-androgen therapy showed no DLT with the selective CXCR2 antagonist, AZD5069, at doses up to 320 mg bid when used in combination with the androgen receptor antagonist enzalutamide up to 160 mg qd.
In this phase I/II multicentre study (NCT03177187), 21 patients were enrolled in a phase I study and 11 patients were enrolled in a phase II study who had received a median six prior lines of therapy as of 10 February 2022. The dual primary endpoint of the study was safety and to establish the recommended phase II dose (RP2D). Results were presented for 15 safety- and response-evaluable patients across four AZD5069 dose levels, from 40 mg bid to 160 mg bid. The doses used were based on previous observations of anti-tumour activity with AZD5069 160 mg bid in combination with enzalutamide, along with the finding of enzalutamide-induced CYP3A4 metabolism of AZD5069 in pharmacokinetic studies.
The main grade 3–4 treatment-emergent adverse event (TEAE), occurring in >10% of patients, was uncomplicated, reversible neutropenia (1/3 at 80 mg bid, 2/3 at 120 mg bid and 4/6 at 160 mg bid), an on-target effect of AZD5069. Other TEAEs (any grade) included fatigue (4/15), nausea (4/15) and anaemia (4/15). No dose-limiting toxicity was reported and no patient discontinued treatment or had a dose reduction because of a treatment-related adverse event.
At AZD5069 doses of 40 mg and 80 mg bid, the maximum plasma concentration (Cmax) and area under the concentration–time curve (AUC) of AZD5069 following enzalutamide administration was significantly reduced. In contrast, at a dose of 160 mg bid, there was no significant difference in AZD5069 Cmax and AUC following enzalutamide administration. The pharmacokinetic parameters of enzalutamide at steady state were comparable to historical data.
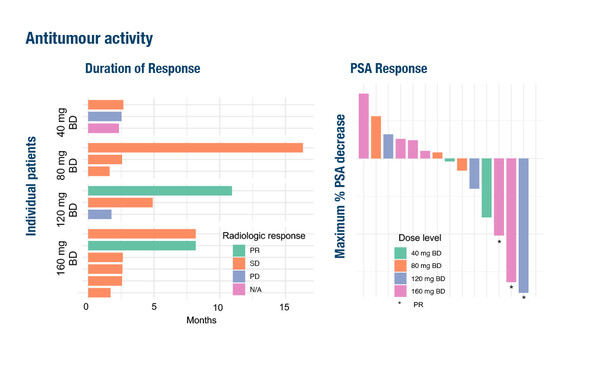
Partial response (PR) was observed in 2/15 response-evaluable patients and 10/15 patients had stable disease (SD) with duration spanning from <2 months to >16 months.
In one patient at the 120 mg bid dose level and two patients at the 160 mg dose level, a substantial reduction in prostate-specific antigen (PSA) level was associated with PRs. In the patient with PR at the AZD5069 120 mg bid dose level, the target lesion was reduced by 31% according to RECIST criteria and PSA was reduced by 89%, with duration of response lasting 11 months. This patient had previously progressed on androgen deprivation therapy, docetaxel and enzalutamide.
The study provides evidence suggesting that CXCR2 blockade of intratumoural myeloid infiltration may reverse endocrine resistance in some patients with castration-resistant mCRPC.
Abstract presented:
Guo C, et al. A Phase (Ph) I/II trial of the CXCR2 antagonist AZD5069 in combination with enzalutamide (ENZA) in patients (pts) with metastatic castration resistant prostate cancer (mCRPC). ESMO Congress 2022, Abstract 454O
Proffered Paper Session – Developmental therapeutics, 10.09.2022, h. 10:15 – 11:45, Orléans Auditorium