Findings show overall survival benefits with front-line atezolizumab versus single-agent chemotherapy, but questions remain regarding who might benefit
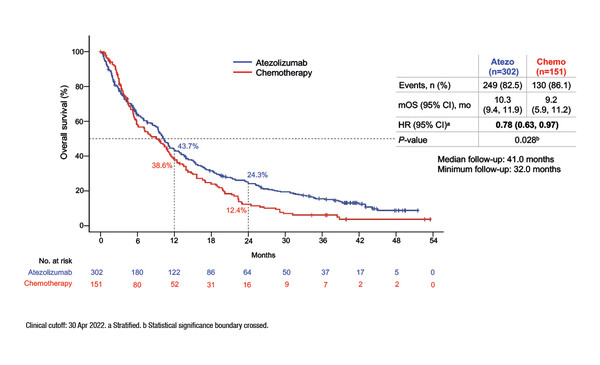
A phase III study of first-line atezolizumab versus single-agent chemotherapy in patients with non-small-cell lung cancer (NSCLC) deemed ineligible for treatment with a platinum-containing regimen revealed a significant survival benefit for the immune checkpoint inhibitor alongside no new or unexpected safety signals (LBA11).
Results from the IPSOS randomised phase III study presented at ESMO Congress 2022 showed that atezolizumab significantly improved median overall survival (OS) versus single-agent chemotherapy (stratified hazard ratio [HR] 0.78; 95% confidence interval [CI] 0.63–0.97; p=0.028), with a consistent benefit seen across key subgroups, including PD-L1 expression levels, performance status (PS) and histology. Patients with locally advanced/metastatic NSCLC without driver mutations who were ineligible for first-line platinum-doublet chemotherapy due to poor PS (≥2) or comorbidities in patients aged ≥70 years were randomised 2:1 to atezolizumab 1200 mg IV q3w or single-agent vinorelbine or gemcitabine in 3- or 4-week cycles.
Commenting on the results, Dr Lizza Hendriks of Maastricht University Medical Center, Netherlands notes that: “It is reassuring because median OS for the atezolizumab arm in this study is similar to historical data from the PePS2 trial of pembrolizumab monotherapy in PS 2 patients with NSCLC (Lancet Respir Med. 2020;8:895–904), so you have confirmation that, at least for select patients with PS ≥2 and those aged ≥70 years with comorbidities, immunotherapy is a good option. I would like to see the full eligibility criteria (e.g. similar to the PePS2 trial, PS 2 but not rapidly deteriorating), but as we know these patients were excluded from pivotal clinical trials, this is really a patient population with a medical unmet need.”
According to Hendriks, this study is important because it is the first randomised clinical trial to evaluate single-agent immunotherapy in poor prognosis patients. In terms of whether this study could be practice changing, she is more cautious. “This is a select patient population and truly a poor performance patient population with 83% having ECOG PS ≥2. We should not be nihilistic in this patient population – for relatively stable patients, immunotherapy could be an option – but we must also remain realistic that it will not work for everyone,” she says.
ESMO clinical practice guidelines state that (elderly) patients with ECOG PS 2 can receive doublet platinum-based chemotherapy (preferably carboplatin) if they are eligible and have adequate organ function (Ann Oncol. 2018;29(Suppl 4):iv192–iv237). Hendriks thinks it is quite difficult to grasp which types of patients were involved in this trial and precisely why they were not eligible for platinum-doublet chemotherapy.
“PS ≥2 can be a result of the cancer itself or of comorbidities, thus it can be observed in a heterogenous group of patients making it more difficult to determine the optimal management strategy. For instance, if you have a rapidly deteriorating patient, it would be quite difficult to obtain benefit from any treatment, including immunotherapy,” she says. “It would be interesting to see details of the tumour burden (low tumour burden is often associated with better outcomes with immunotherapy) and comorbidities of patients involved in the trial or how stable they were in terms of PS prior to study enrolment. Only patients with a life expectancy of ≥8 weeks were eligible, and these are not patients with a PS ≥2 that is rapidly deteriorating. Furthermore, only patients without severe cardiac comorbidities and with adequate haematological and end organ function were eligible, so for me it is not clear why these patients were not eligible for carboplatin-doublet chemotherapy. Benefit was seen across PD-L1 subgroups, yet we know that single-agent immunotherapy works better in patients with higher PD-L1 levels. To better understand how these findings will impact clinical practice, we need to identify the patients who will benefit, which could be those with relatively stable PS and maybe higher PD-L1 or relatively low tumour burden, or those with a poor PS due to comorbidities and not due to the NSCLC. We also need to evaluate biomarkers other than PD-L1, such as circulating tumour DNA (ctDNA) as from other studies we know that those without detectable ctDNA at baseline, or with ctDNA that becomes undetectable during immunotherapy, have the best outcomes,” concludes Hendriks.
Abstract presented:
Lee SM, et al. IPSOS: Results from a phase 3 study of first-line (1L) atezolizumab (atezo) vs single-agent chemotherapy (chemo) in patients (pts) with NSCLC not eligible for a platinum-containing regimen. ESMO Congress 2022, LBA11
Presidential Symposium III, 12.09.2022, h. 16:30 – 18:15, Paris Auditorium