Results from the RADICALS-HD and PRESTO trials show encouraging metastasis-free and biochemical progression-free survival benefits with androgen deprivation therapy, but they are not practice-changing yet
Data from two phase III trials evaluating the use of androgen deprivation therapy (ADT) alongside radiotherapy in post-operative patients with prostate cancer were presented at ESMO Congress 2022.
The first results from the RADICALS-HD trial in patients undergoing post-operative radiotherapy after radical prostatectomy (RP; n=2839) showed longer metastasis-free survival (MFS), the primary endpoint (hazard ratio [HR] 0.77, 95% confidence interval [CI] 0.61–0.97); 72% versus 78% at 10 years), and time to salvage ADT (HR 0.73, 95% CI 0.59–0.91) in those who received 24 months versus 6 months of ADT (LBA9). Overall survival (OS) was not improved (HR 0.88, 95% CI 0.66–1.17). Six months of ADT versus no ADT improved time to salvage ADT but not MFS. Pre-planned subgroup analyses revealed favourable MFS outcomes for 24 months versus 6 months of ADT regardless of pre-radiotherapy prostate-specific antigen (PSA) level (<0.3 ng/mL, 0.3–0.5 ng/mL and >0.5 ng/mL) or Charlson comorbidity index (0, 1 and ≥2).
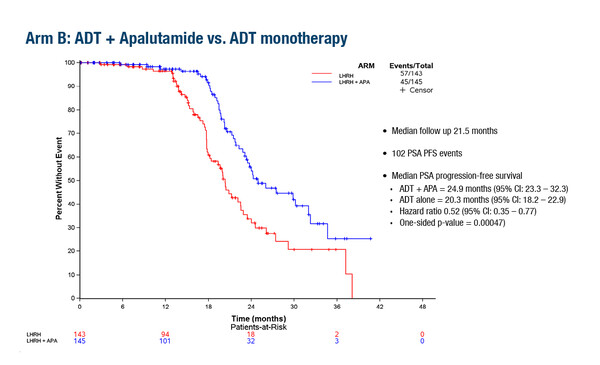
In a second study, the PRESTO trial, the first planned interim analysis showed that both ADT + apalutamide (APA; n=168) and ADT + APA + abiraterone acetate plus prednisone (AAP; n169) significantly prolonged the primary endpoint of biochemical progression-free survival (bPFS; serum prostate-specific antigen [PSA] >0.2 ng/mL following treatment), versus ADT alone (n=166), in patients with high-risk biochemically relapsed prostate cancer following RP (LBA63).
Median bPFS was 24.9 months for ADT + APA versus 20.3 months for ADT (HR 0.52; 95% CI 0.35–0.77) and 26.0 months for ADT + APA + AAP versus 20.0 months for ADT (HR 0.48; 95% CI 0.32–0.71). Median time to testosterone recovery (>50 ng/dL) was 4.0 months for ADT, 3.9 months for ADT + APA and 4.8 months for ADT + APA + AAP. Hypertension was the most common grade ≥2 adverse event, and was more frequent in the ADT + APA + AAP arm (30.4%) than in the ADT + APA (23.4%) and ADT arms (19.4%).
Findings from the DADSPORT meta-analysis of results from NRG/RTOG 9601, GETUG-AFU 16, NRG/RTOG 0534 and RADICALS-HD were also presented, showing 6 months of hormone therapy improved MFS (HR 0.82; 95% CI 0.70–0.96; p=0.01) but not OS in men receiving radiotherapy following RP for localised prostate cancer after a median follow-up of ≥8 years (LBA64). There was no clear evidence showing that hormone therapy improved OS, regardless of whether 6 months or 24 months of hormone therapy was given.
Taken together, these findings were welcomed by Prof. Stephane Supiot, from Institut de Cancérologie de l’Ouest and Université de Nantes, France, as particularly relevant for high-risk biochemically-relapsing patients: “Biochemical failure following surgery is still common and most relapses are local, although a PSA doubling time of less than 6 months is a recognised risk factor for developing metastases (Urol Int. 2018;100:251–262). Radiotherapy is routinely used for biochemically-relapsing patients, but its efficacy is limited to localised relapses, such as in the prostate bed or pelvic lymph nodes.” Supiot stresses the importance of these findings for patients receiving radiotherapy: “The trial results further support the benefit of adding ADT for patients undergoing radiotherapy, but the lack of OS benefit of immediate ADT as shown in the DADSPORT meta-analysis (LBA64) can be interpreted that delayed ADT can salvage patients at the metastatic stage. More work is needed to determine the optimal treatment duration and whether we should combine luteinising hormone-releasing hormone (LH-RH) agonists with androgen receptor targeting drugs.”
According to Supiot, the PRESTO trial is not yet practice-changing. “A longer follow-up will also determine if the bPFS benefit in PRESTO translates into clinical benefit in terms of MFS,” he adds. “The benefit of adjuvant radiotherapy after RP in patients with pT3N0 prostate cancer has already been demonstrated, for example in the EORTC 22911 trial, which showed a 10-year bPFS rate of 60.6% in patients who received radiotherapy versus 41.1% in those following a wait-and-see approach (p<0.0001), although OS did not differ between the groups (Int J Radiat Oncol Biol Phys. 2010;78(Suppl S29):Abstract 62). The addition of antiandrogen therapy was then reported to improve the 12-year OS rate versus radiation therapy alone in the RTOG 9601 trial of men with T2 or T3 prostate cancer (12-year OS 76.3% versus 71.3%, respectively; HR 0.77; 95% CI 0.59–0.99; p=0.04), with greater gains in OS and MFS observed in patients with higher PSA levels at study entry (N Engl J Med. 2017;376:417–428). However, in men receiving early salvage radiotherapy (PSA ≤0.6 ng/mL) in RTOG 9601, post-hoc analyses found long-term antiandrogen treatment was not associated with improved OS due to an increased combined odds of grades 3 to 5 cardiac and neurological events (JAMA Oncol. 2020;6:735–743). Longer follow-up data from RADICALS-HD and PRESTO are also needed for a full toxicity assessment.
“These data are encouraging and longer follow-ups and further study should give additional information regarding the optimal treatment duration and treatment combinations, considering toxicity and quality of life alongside efficacy data,” concludes Supiot. “The toxicity of AAP and APA is well known and generally manageable, but care is needed in patients with cardiovascular risk factors. Patient benefit may vary according to characteristics such as age, high-risk disease and cardiovascular comorbidities. Other research is also examining prognostic biomarkers in prostate cancer, which may guide patient selection and tailored treatment approaches (J Clin Oncol. 2022;40(Suppl):222; NCT03371719).”
Abstracts presented:
Parker C, et al. Duration of androgen deprivation therapy (ADT) with post-operative radiotherapy (RT) for prostate cancer: first results of the RADICALS-HD trial (ISRCTN40814031). ESMO Congress 2022, LBA9
Presidential III, 12.09.2022, h. 16:30 – 18:15, Paris Auditorium
Aggarwal R, et al. PRESTO: A phase 3, open-label study of androgen annihilation in patients (pts) with high-risk biochemically relapsed prostate cancer (AFT-19) ESMO Congress 2022, LBA63
Proffered Paper Session – GU tumours, prostate, 11.09.2022, h. 08:30 – 10:00, Antibes Auditorium
Burdett S, et al. Duration of androgen suppression with post-operative radiotherapy (DADSPORT): A collaborative meta-analysis of aggregate data. ESMO Congress 2022, LBA64
Mini Oral Session – GU tumours, prostate, 11.0922, h. 10:15 – 11:45, Quimper Auditorium