Results from the COSMIC-313 trial show that adding cabozantinib to nivolumab plus ipilimumab prolongs progression-free survival, but also increases toxicity
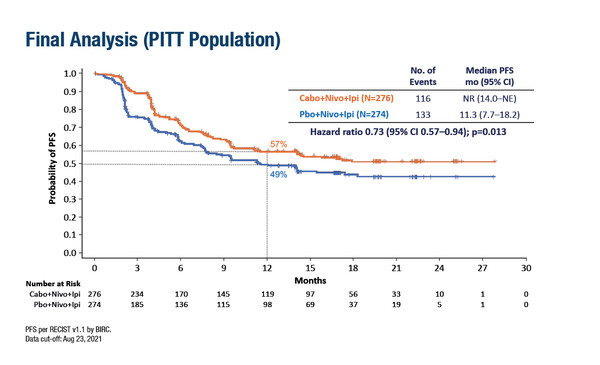
Cabozantinib plus nivolumab plus ipilimumab (C+N+I) led to a significant 27% reduction in the risk of progression compared with N+I among 550 patients with untreated intermediate- or poor-risk advanced renal cell carcinoma (RCC) (hazard ratio [HR] 0.73; 95% confidence interval [CI] 0.57–0.94; p=0.013), according to Late-Breaking Abstract results from the COSMIC-313 trial presented at ESMO Congress 2022 (LBA8). Median progression-free survival (PFS) was not reached with C+N+I (95% CI 14.0–not estimable) and was 11.3 months (95% CI 7.7–18.2) for N+I [ADD FIGURE HERE]. Objective response rates were 43% and 36% with the triplet and doublet combinations, respectively, and the median duration of response was not reached in either treatment arm.
In this global, double-blind, randomised trial, patients with clear cell advanced RCC received N+I every 3 weeks for 4 cycles followed by N every 4 weeks for up to 2 years. Randomisation to cabozantinib or placebo was stratified according to region and disease risk and PFS was assessed by blinded independent review.
Meeting its primary endpoint, COSMIC-313 is the first trial to show that adding a TKI to dual checkpoint inhibitor therapy can improve PFS in this setting. But are the results practice changing?
“The findings are certainly exciting and important,” says Prof. Viktor Grünwald from University Hospital Essen, Germany. “What is really missing at the moment is therapy that will provide complete and durable responses with improved long-term outcomes for our patients. The triplet seems to prevent early progression, which is a major advantage over the nivolumab + ipilimumab doublet.” However, the expert is more cautious regarding the practice-changing nature of the data. “Firstly, we will need to wait to see what the overall survival data show,” he says. “And, of course, efficacy data will have to be balanced alongside the additional toxicity of the triplet regimen, which shows quite a high burden of severe side-effects and toxicity-related treatment discontinuations.”
In the COSMIC-313 trial, grade 3–4 treatment-related adverse events (TRAEs) occurred in 73% of patients receiving the triplet combination compared with 41% receiving the doublet combination. In addition, there were more than twice the number of TRAEs leading to discontinuation of all treatment components with the triplet compared with the doublet regimen (12% versus 5%, respectively). Elevated liver enzymes occurred in more patients receiving the triplet combination compared with the doublet combination. Three patients in both treatment arms had grade 5 TRAEs: gastrointestinal haemorrhage, hepatic failure and respiratory failure in the triplet combination arm and renal failure, myocarditis and sudden death in the doublet combination arm.
Grünwald says that toxicity data from COSMIC-313 appear to mirror results revealed by an exploratory analysis of the discontinued C+N+I arm of the CheckMate 9ER trial in 50 patients with advanced RCC presented earlier this year (International Kidney Cancer Symposium 2022, Abstract). Because of this, he thinks that more information on the toxicity profile in COSMIC-313 is needed to inform the future role of the triplet regimen in treatment. “In CheckMate 9ER, dose modifications due to toxicity were fairly common and 40% of patients had at least one grade 3–4 immune-mediated hepatic adverse event. We need to understand to what extent the triplet's dose reductions and discontinuations affected treatment outcomes in COSMIC 313,” he concludes.
Abstract presented:
Choueiri T, et al. Phase 3 study of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in previously untreated advanced renal cell carcinoma (aRCC) of IMDC intermediate or poor risk (COSMIC-313). ESMO Congress 2022, LBA8
Presidential Symposium III, 12.09.2022, h. 16:30 – 18:15, Paris Auditorium