Prospective evidence from a multicentre study support the need for a more personalised immuno-oncology approach based on sex and gender differences
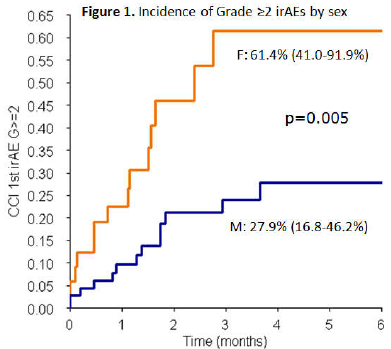
Results from an ongoing multicentre, observational, prospective study presented yesterday at the ESMO Congress 2021 show that cumulative grade ≥2 immune-related adverse events (irAEs) in patients receiving immune checkpoint inhibitors were reported more frequently in females (n=41) than in males (n=65) (Abstract 1795P).
Women receiving immunotherapy may benefit from personalised and closer follow-up
The findings come as no surprise to Dr Berna Özdemir from Bern University Hospital (Inselspital), Switzerland, and member of the ESMO Gender Medicine Task Force. “They confirm indications from retrospective data, showing a higher incidence of these types of events in the female population,” she says. “Recognition and appropriate management of irAEs at an early stage is important in ensuring optimum patient treatment. The data from this study send a signal to oncologists that women receiving immunotherapy may benefit from personalised and closer follow-up,” advises Özdemir.
The likely drivers of the inter-sex disparities in irAEs are the well-documented biological differences in their immune system reactions, with women tending to have stronger immune responses than men, independent of age and ethnicity. However, differentiating the impact of sex from that of gender is fraught with difficulty, given the constant interplay between the two. In general, clinical trials have not been powered to investigate sex and gender differences. But as their role in side -effects, and probably response, to therapy becomes increasingly important, so does the need to revisit clinical trial designs to incorporate sex and gender. Among 106 patients included in the study to date – with a minimum follow-up of 12 months – the 6-month cumulative incidence of grade ≥2 irAEs in females was more than double that in males (61.4% versus 27.9%; p=0.005). The difference remained significant in multivariate analysis, adjusting for cancer type, ECOG performance status and setting (p=0.001). However, of the 106 irAEs of any type and grade reported, fewer were reported in females than in males (38.7% vs 61.3%). Patients living alone, particularly males, were at a higher risk of developing grade ≥2 irAEs compared with those not living alone.
The study authors aim to use the final trial results to develop tools enabling the prediction of irAE risk based on sex and gender determinants. Özdemir thinks the initial results are encouraging for personalising immunotherapy. “The results are a more general indication that immunotherapy selection in the future should take account not only of the type of cancer but also the sex of the patient,” she concludes. “Women may benefit more from other agents that modulate the immune system, alone or in combination with other therapies, whereas men appear to respond better than women to the current checkpoint inhibitors.”
The study, conducted at the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy, Karolinska University Hospital, Solna, Sweden, St Vincent's University Hospital, Dublin, Ireland and Oslo University Hospital, Norway, is estimated to complete in 2023.
Miceli R et al. Gender difference in side effects of immunotherapy: a possible clue to optimize cancer treatment. ESMO Congress 2021, Abstract 1795P