A Japanese study reports discrepancies among centres, highlighting the need for better strategies to improve the quality of treatment recommendations
There is an emerging need for strategies to improve the quality of tumour board outputs, as highlighted by a prospective analysis presented yesterday at the ESMO Congress 2021, which reported poor concordance between consensus treatment recommendations and those of multidisciplinary tumour boards (MTBs), particularly for tumours with poorly defined therapy options (Abstract 519MO).
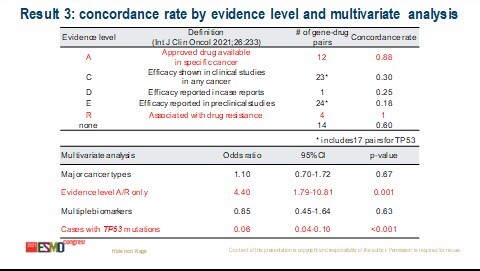
Overall, fewer than two-thirds (62%) of MTBs from 12 leading cancer centres in Japan showed agreement with consensus management recommendations for 50 simulated cancer cases, according to study results. The MTBs were asked to independently recommend treatment for simulated cases that incorporated frequently observed genomic alterations. The results were compared to consensus recommendations developed according to Japanese practice guidelines.
Among 600 MTB treatment recommendations, high concordance was observed for cases of colorectal cancer (100%), those with ROS1 fusion (100%) and those with a high level of evidence for recommendations (A/R 88%/100%). However, concordance was extremely low for cervical cancer (11%), cases with TP53 mutation (16%), and those with a low level of evidence for recommendations (C/D/E 30%/25%/18%). In multivariate analysis, a high level of evidence was associated with a four-fold increase in the chance of concordance (odds ratio [OR] 4.4; 95% confidence interval [CI] 1.8–10.8), whereas a TP53 alteration with a much lower chance (OR 0.06; 95% CI 0.04–0.10).
“The low concordance observed for cervical cancer and TP53 mutation is probably due to the fact that therapeutic strategies are not well defined for patients with these types of tumours,” says Prof. Ulrik Lassen from Rigshospitalet, Copenhagen, Denmark. “When there is a very obvious treatment choice, there will be greater agreement on the management approach.” The degree of concordance will also depend on differences, or similarities, between the individual centres in the platforms used, the levels of skill and experience of the MTB members and the numbers of patients the teams have treated.
Virtual tumour board meetings provide a digital solution to aligning treatment decisions across centres, thus opening up opportunities for collaboration among bigger and smaller centres. “Several sites can review cases together but on a remote basis. This provides a great learning environment and helps small centres to gain knowledge from the bigger centres that have more experience by virtue of the greater numbers of patients they treat, often across a range of tumour types,” says Lassen. In Denmark, a national virtual tumour board meeting involving seven centres is held every week. “Of course, it is easier in a small country like Denmark and when you have fewer patients,” admits Lassen, “but this approach should also be achievable on a larger scale.”
Lassen expects fairly rapid progress to be made in improving MTB decision-making, a process that will be facilitated further by factoring artificial intelligence (AI) into the equation. “Algorithms keep getting better and the amount of knowledge, and the rate at which it is entering shared fields, is greater now than ever before. In addition, more centres now have access to advanced genomic sequencing platforms. While AI is not yet ready for widespread use in clinics, it will be in the near future. However, AI cannot be used in isolation, there will always be the need for human interpretation to validate the findings.”
Kage H et al. Concordance analysis of treatment recommendations between central consensus and multidisciplinary tumor boards. ESMO Congress 2021, Abstract 519MO
Mini oral session – Developmental therapeutics, 20.9.2021, h. 18:10 – 18:15, Channel 3