Significant, but clinically modest, prolongation of radiographic progression-free survival and event-free survival was achieved with darolutamide in the SAKK 08/16 study
Results from a proof-of-concept trial, presented as a Late-Breaking Abstract today at the ESMO Congress 2021, suggest that switch maintenance with darolutamide may be a valuable new concept in the treatment of metastatic castration-resistant prostate cancer (mCRPC) (LBA26).
The double-blind phase II SAKK 08/16 study involved 92 randomised patients with mCRPC, prior therapy with novel hormonal agents (NHA; abiraterone and/or enzalutamide) and non-progressive disease on taxane-based chemotherapy (docetaxel ≥300 mg/m2 or cabazitaxel ≥80 mg/m2). Patients received darolutamide 600 mg or placebo twice daily 2–8 weeks after the end of the chemotherapy. Median follow-up was 18 months.
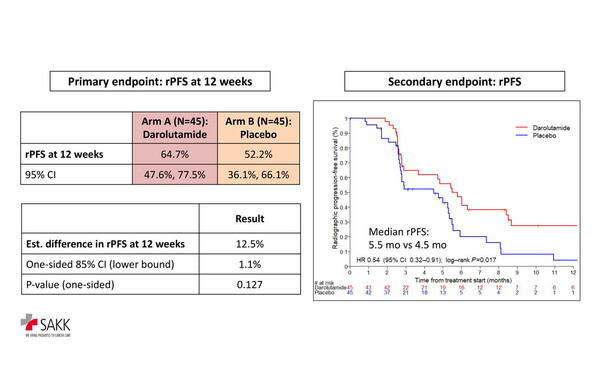
The primary endpoint of radiographic progression-free survival (rPFS) at 12 weeks was significantly improved with darolutamide (64.7%) compared with placebo (52.2%; p=0.127, below significance level of 0.15). There was significant prolongation of median rPFS with darolutamide than placebo (5.5 months versus 4.5 months; hazard ratio [HR] 0.54; 95% confidence interval [CI] 0.32–0.91; p=0.017) and median EFS (5.4 months versus 2.9 months; HR 0.46; 95% CI 0.29–0.73; p<0.001). Prostate-specific antigen 50% response occurred in 22% of patients receiving darolutamide but only 4% of those receiving placebo. No significant difference in overall survival was observed between darolutamide and placebo (24 months versus 21.3 months; HR 0.62; 95% CI 0.3–1.26; p=0.181).
Regarding tolerability, treatment-related adverse events were mild and similar with darolutamide and placebo: grade 1, 26% versus 22%; grade 2, 13% versus 15%; grade 3, both 2%. Grade 1/2 fatigue was less common with darolutamide than placebo (11% versus 20%).
Cathomas R. Darolutamide maintenance in metastatic castration resistant prostate cancer (mCRPC) previously treated with novel hormonal agents (NHA) and non-progressive disease after subsequent treatment with a taxane: A randomized double-blind placebo-controlled phase II trial (SAKK 08/16). ESMO Congress 2021, LBA26
Mini oral session – Genitourinary tumours, prostate 19.9.2021, h. 17.35 –17.40, Channel 2