Results from the SAKK 01/10 trial demonstrate favourable progression-free survival and low toxicity with single-dose carboplatin and involved-node radiotherapy
Low-dose chemotherapy with carboplatin and a reduced targeted irradiated volume is a favourable approach in seminoma, particularly in stage IIA, according to findings from the SAKK 01/10 trial (LBA30) presented today at the ESMO Congress 2021.
Standard treatment options for clinical stage IIA and IIB seminoma currently include either extensive ‘dog leg’ paraaortal/pelvic radiotherapy or intensive chemotherapy (4 cycles of etoposide and cisplatin or 3 cycles of bleomycin, etoposide and cisplatin). While both treatment modalities are associated with excellent efficacy, there is a high rate of short- and long-term toxicities, including risk of second cancers.
The single-arm phase II SAKK 01/10 trial investigated the efficacy and safety of a de-escalation strategy involving 1 cycle of carboplatin AUC7 followed by involved-node radiotherapy (30 Gy in stage IIA and 36 Gy in stage IIB) in 116 patients with stage IIA/B seminoma (de novo or relapsed on active surveillance).
“Data from this large multicentre, prospective trial confirm our previous findings from a single-centre pilot study (Ann Oncol. 2013;24(8):2104-7) and from the TRISST trial (J Clin Oncol 39, 2021 (suppl 6; abstr 374)),” says Prof. Robert Huddart from the Institute of Cancer Research, Sutton, UK, commenting on the results.
Over a median follow-up of 4.5 years, the primary endpoint of 3-year progression free survival (PFS) was 93.7% (90% confidence interval [CI] 88.5– 96.6). By stage, 3-year PFS was 95.2% (90% CI 85.5–98.5) in patients with stage IIA and 92.6% (90% CI 85.2–96.4) with stage IIB. One patient with stage IIA and 6 patients with stage IIB developed a recurrence, all of which were outside the radiotherapy volumes and were salvaged with conventional chemotherapy.
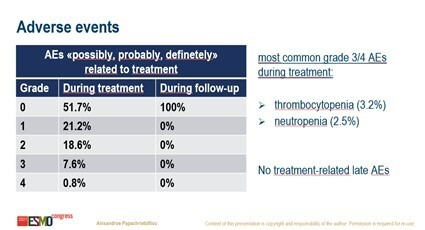
The study authors described the rate of adverse events as ‘very low’ (Figure). Although the long-term risks associated with the chemo-radiotherapy combination are as yet unknown, Huddart believes the regimen is generally well tolerated and associated with less toxicity than would be expected with standard chemotherapy regimens.
Prof. Huddart explains that the situation with stage IIB seminoma is more ‘nuanced’, but believes the carboplatin–radiotherapy regimen could still be an important option. “Recurrence in 6 patients with stage IIB was noted but most patients were still cured by this approach. As recurrences were observed only outside the radiotherapy volumes, positron emission tomography may help to improve tumour localisation and targeting of the radiotherapy.” He continues, “Another advantage of using the chemo–radiotherapy combination in the first instance is that any recurrence can subsequently be managed with standard chemotherapy.”
Considering how de-escalated therapy could be adapted further, Prof. Huddart says, “It may be possible to reduce the radiotherapy dose from 36 Gy to 30 Gy in stage IIB tumours as used in other studies of this approach. Given the success of BEP x 1 in high-risk non-seminomatous germ-cell tumours (NSGCT), using etoposide and cisplatin instead of carboplatin in the combination may be preferred by some oncologists, but would result in greater toxicity that many patients may wish to avoid. Alternatively, the combination of surgery with or without carboplatin is being investigated in selected patients with stage IIA tumours; however, the recurrence risk without carboplatin may be higher than with involved-node radiotherapy.”
Prof. Huddart acknowledges that comparative data investigating different treatment arms would be valuable, “However, randomised controlled trials are impractical, as stage IIA and IIB seminoma is relatively uncommon and primary endpoint selection is difficult – patients survive for many years, so we are interested in the impact of treatment on quality of life and how to minimise toxicity. These robust new data are a step forward.”
Papachristofilou A et al. Single-dose carboplatin followed by involved-node radiotherapy as curative treatment for seminoma stage IIA/B: efficacy results from the international multicenter phase II trial SAKK 01/10. ESMO Congress 2021, Abstract LBA30
Proffered Paper session – Genitourinary tumours, non-prostate 2, 19.9.2021, h. 14:20 – 14:30, Channel 2