Manageable toxicity profile and signs of clinical benefit seen with gavocabtagene autoleucel in treatment-refractory mesothelin-expressing solid tumours
Chimeric antigen receptor (CAR)-engineered T-cell therapies have shown limited benefit in solid tumours so far. Results presented today at ESMO Congress 2021 suggest that the T-cell receptor fusion construct gavocabtagene autoleucel (gavo-cel; TC-210) may offer an alternative approach for exploring the use of T-cell therapy in certain solid tumours (Abstract 959O).
Gavo-cel consists of autologous genetically engineered T-cells expressing a single-domain anti-mesothelin antibody fused to the CD3-epsilon subunit of the T-cell receptor. The construct integrates into endogenous T-cell receptor complexes, allowing HLA-independent T-cell engagement to mesothelin on cancer cells.
In the dose-escalation part of an ongoing phase I/II trial, 17 patients (12 with malignant mesothelioma [MPM], 4 with ovarian cancer and 1 with cholangiocarcinoma) received a single IV dose of gavo-cel. The dose levels (DLs) investigated were 5x107 cells/m2 alone (DL 0, n=1) or after lymphodepletion (DL1, n=6), 1x108 cells/m2 alone (DL2, n=1) or after lymphodepletion (DL3, n=5), and 5x108 cells/m2 alone (DL4, n=1) or after lymphodepletion (DL5, n=3). Patients had received a median of five (range, 1–9) prior therapies, including immune checkpoint inhibitor (ICI) (65%) and anti-mesothelin therapies (29%).
There were two dose-limiting toxicities (grade 3 pneumonitis at DL1 and haemorrhage at DL5) and six events of grade ≥3 cytokine release syndrome.
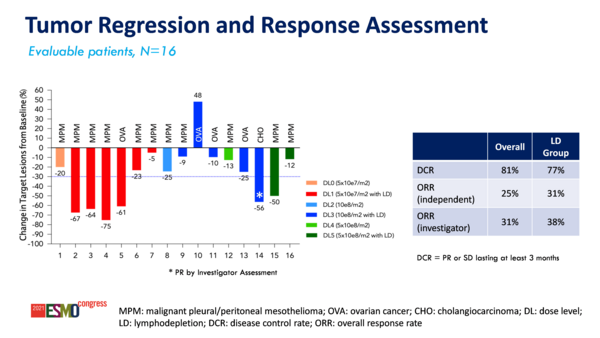
Following gavo-cel treatment, six out of 16 evaluable patients had ≥30% decrease from baseline in target lesions and the disease control rate was 81%. The objective response rate was 25%, which included 1 patient with MPM who had a partial response and complete metabolic response by positron emission tomography. As of the data cut-off date of June 30, 2021, median progression-free survival was 177 days and median overall survival was 337 days.
Translational data demonstrated that the median time to peak gavo-cel expansion was 15.5 days (median, 854.9 copies/μg DNA) at DL1, 7 days (median, 14539.7 copies/μg DNA) at DL3 and 9 days (median, 5275.8 copies/μg DNA) at DL5. A decrease in soluble mesothelin-related peptides and serum megakaryocyte potentiating factor levels were observed post infusion among patients with detectable baseline levels.
Further dose testing is ongoing to determine the recommended phase II dose of gavo-cel, which could also be combined with pembrolizumab for patients with lung cancer.
Hong D et al. Gavocabtagene autoleucel (gavo-cel, TC-210) dose escalation in refractory mesothelin-expressing solid tumors. ESMO Congress 2021, Abstract 959O
Proffered Paper session – Investigational immunotherapy, 17.09.2021, 14:30 – 14:30, Channel 4