Nearly 1 in 4 patients with pleural malignancy continue to benefit from nivolumab plus ipilimumab at 3 years
Updated data from the phase III CheckMate 743 study confirm the place of immunotherapy versus chemotherapy as the first-line therapy of choice in malignant pleural mesothelioma (MPM). Findings will be presented at the ESMO Congress 2021 taking place virtually on 16-22 September.
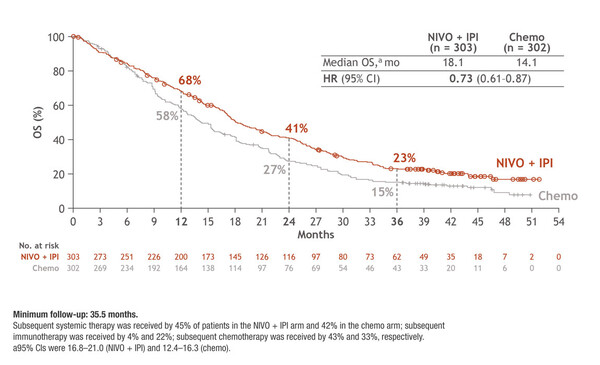
“These data are incredibly important for mesothelioma because we can see the trend for a long-term benefit of immunotherapy in patients with this aggressive, difficult-to-treat malignancy,” says Dr Alessandra Curioni Fontecedro from the University Hospital of Zurich, Switzerland, and Editor-in-Chief of the ESMO Daily Reporter, commenting on the results. In CheckMate 743, the 3-year overall survival (OS) rate for nivolumab plus ipilimumab was 1.5-times that of chemotherapy (23.2% versus 15.4%) in patients with unresectable MPM, after a minimum follow-up of 35.5 months and patients being off therapy for 1 year (LBA65). Among more than 600 patients, those receiving immunotherapy (n=303) had a 27% reduction in the risk of death compared with those receiving chemotherapy (hazard ratio 0.73, 95% confidence intervals 0.61–0.87) (Figure).
The overall response rate (ORR) was 39.6% with nivolumab plus ipilimumab and 44.0% with chemotherapy. Exploratory biomarker analysis revealed that a high four-gene inflammatory gene signature score (CD8A, PD-L1, STAT-1, LAG-3) was associated with longer OS in patients receiving immunotherapy but not in those receiving chemotherapy.
“The survival benefit of nivolumab plus ipilimumab was observed despite a higher ORR with chemotherapy. What is more, the 3-year duration-of-response rate was 28% among patients receiving immunotherapy but 0% in those receiving chemotherapy,” continues Curioni Fontecedro.
Earlier results from the study changed the first-line standard-of-care treatment for patients with MPM in several countries around the world
The findings suggesting an association between the inflammatory gene signature score and OS in patients receiving immunotherapy are encouraging. “However, the results from this study are not yet sufficiently clear to support immune gene signature as a predictive biomarker for immunotherapy,” she stresses. “Further investigation of this and of other molecular features of mesothelioma will be fundamental to our understanding of which patients might still receive chemotherapy. For now, there are no biomarkers that can be used as standard of care in the clinical setting.”
The US FDA approval in 2020 of nivolumab plus ipilimumab for previously untreated, unresectable MPM was based on the initial report of the CheckMate 743 study, which showed an OS advantage for immunotherapy over chemotherapy at median follow-up of 29.7 months. “Earlier results from the study changed the first-line standard-of-care treatment for patients with MPM in several countries around the world,” says Curioni Fontecedro. “These updated data further reinforce the previously published findings and support the benefit of giving patients immunotherapy instead of chemotherapy.”
Peters S et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) vs chemotherapy (chemo) in patients (pts) with unresectable malignant pleural mesothelioma (MPM): 3-year update from CheckMate 743. ESMO Congress 2021, LBA65
Proffered Paper session – Non-metastatic NSCLC and other thoracic malignancies. 17.9.2021, h. 13:40 – 13:50, Channel 2