Results from two studies suggest that withholding chemotherapy for some patients may not impact efficacy, but an understanding of clinical and biological features is paramount
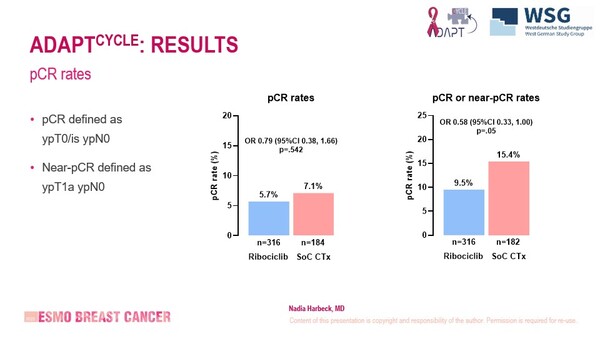
The role of chemotherapy is still uncertain in some subgroups of patients with early-stage breast cancer and results from the neoadjuvant cohort of the ADAPTcycle trial revealed similar and low pathological complete response (pCR) rates of 5.7% with ribociclib and 7.1% with chemotherapy (odds ratio 0.79; 95% confidence interval [CI] 0.38–1.66; p=0.542) as presented at ESMO Breast Cancer 2025 (Munich, 14–17 May) (Abstract 189O).
The ADAPTcycle trial is comparing ribociclib plus endocrine therapy versus chemotherapy (followed by adjuvant endocrine therapy) in patients with early breast cancer and intermediate-risk oestrogen receptor (ER)-positive/HER2-negative disease and no endocrine therapy response or intermediate/high-risk disease and response to endocrine therapy (NCT04055493). This analysis involved 554 patients who had received up to 12 months of neoadjuvant endocrine-based therapy, with mixed baseline tumour characteristics, including lower-risk disease but higher-risk genomic markers and a high clinical risk but lower genomic risk. Risk and endocrine response were determined by clinical factors, Oncotype DX and Ki-67.
Interestingly, pCR rates with ribociclib versus chemotherapy were 6.1% versus 7.4% for patients with no or minimal node involvement (cN0–1), 8.0% versus 7.6% for patients with recurrence score >25 and 6.1% versus 7.5% for patients with response to endocrine therapy (ET). Nodal disease involvement was predictive of pCR in a multivariate analysis, whereas study arm, recurrence score and grade were not. Treatment discontinuation was more common in the ribociclib arm than in the chemotherapy arm (19.1% and 11.7%, respectively), as was grade ≥3 neutropenia (29.8% and 14.4%, respectively).
“The similar pCR rates with clinically lower-risk disease suggests that we could potentially forgo chemotherapy and just administer ribociclib to these patients. The analysis also highlights that determining a patient’s risk of recurrence based on clinical and biological characteristics is informative,” says Prof. Christos Sotiriou from Institut Jules Bordet, Brussels, Belgium. “However, randomisation is not yet complete and while pCR data are useful, invasion-free survival and overall survival are needed before we can make firm conclusions.”
Other findings presented at the Congress provided support for gene expression-based multiparameter assay (MPA) to guide chemotherapy use. Results for 387 patients from the feasibility phase (OPTIMA prelim) of the ongoing OPTIMA trial (ISRCTN42400492) in women with resected ER-positive/HER2-negative breast cancer and ≤9° axillary node involvement showed that the 10-year invasive breast cancer-free survival rate was 80% for patients who received standard chemotherapy plus ET and 78% for patients who received MPA-directed treatment (hazard ratio 1.18; 95% CI 0.75–1.83) (Abstract 190O). The MPA-directed strategy was based on the Oncotype DX recurrence score: >25 instigated use of standard therapy and ≤25 instigated ET only.
“These results, showing no difference in invasive-free survival, validate the benefit of using the recurrence score prior to therapy. MPAs capture two key factors about a tumour – cell proliferation status and luminal phenotype (or ER status) – which provide information on the potential for chemotherapy response since chemotherapy is reliant on actively proliferating cells and low ER expression,” says Sotiriou. The researchers also compared invasive-free survival by pre-treatment recurrence risk scores using the Oncotype DX assay and the Prosigna Risk of Recurrence assay. However, Sotiriou cautions against drawing definitive conclusions from these comparisons owing to the low patient numbers and the fact that both assays capture slightly different tumour aspects.
Results are expected in 2026 from the phase III OPTIMA trial, which may add to the mounting evidence from other studies, such as MINDACT (Lancet Oncol. 2021;22:476–488), about the importance of stratifying patients with early-stage breast cancer by recurrence risk so that chemotherapy is withheld from those who are unlikely to benefit.
Programme details
Harbeck N, et al. Ribociclib + endocrine treatment vs SoC chemotherapy in intermediate risk HR+/HER2- early breast cancer: Results from the neoadjuvant cohort of the phase-III WSG ADAPTcycle - trial. ESMO Breast Cancer 2025, Abstract 189O
Proffered Paper Session 2, 15.05.2025, h. 14:00 – 15:30, Room 14
Stein RC, et al. Clinical outcomes in the OPTIMA prelim (Optimal Personalised Treatment of early breast cancer using Multi-Parameter Analysis) feasibility study. ESMO Breast Cancer 2025, Abstract 190O
Proffered Paper Session 2, 15.05.2025, h. 14:00 – 15:30, Room 14