A timing window for testing ESR1 mutations in the blood was identified
For some breast cancer markers, such as ESR1 mutations, circulating tumour (ct)DNA is now considered to be the preferred medium for testing, and findings presented at ESMO Breast Cancer 2025 (Munich, 14–17 May) add to current knowledge on how to use liquid biopsy to refine tumour management.
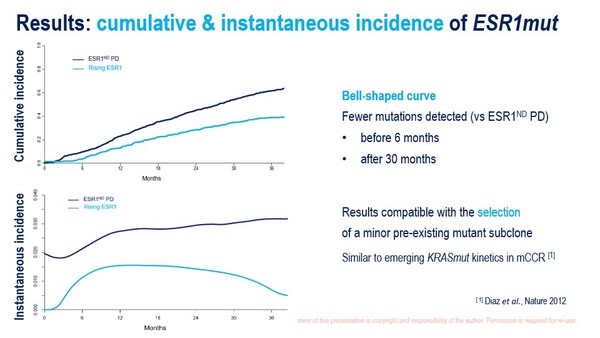
Previously, the phase III PADA-1 trial highlighted the clinical benefit of early blood ESR1-mutation (bESR1mut) therapeutic targeting in 1,017 patients receiving an aromatase inhibitor and palbociclib for the first-line treatment of hormone receptor (HR)-positive/HER2-negative metastatic breast cancer (Lancet Oncol. 2022;23:1367–1377). A secondary analysis of PADA-1 at ESMO Breast Cancer 2025 defined a possible window for ESR1 mutation detection (Abstract 1O). Prospective bESR1mut monitoring revealed a cumulative incidence of 41.7% (in 678 patients with or without radiological disease progression), with an uneven distribution characterised by few detections during the first 6 months of treatment and a reduced incidence after 30 months.
Baseline factors independently associated with the relative incidence of bESR1mut (with or without radiological disease progression) versus disease progression without bESR1mut included: bone metastases (bone only, odds ratio [OR] 2.67; bone plus other sites, OR 2.08; both versus no bone metastases), oestrogen receptor expression (per 10% increase, OR 1.12), age (per 10-year decrease, OR 0.79) and elevated baseline lactate dehydrogenase (OR 1.58).
“Testing for ESR1 mutations in patients receiving first-line aromatase inhibitor and CDK 4/6 inhibitor therapy is on the brink of becoming a standard of care in metastatic breast cancer, so identifying who, when and how often to test are critical questions for doctors to think about in their daily practice,” says Prof. Nicholas Turner from The Royal Marsden NHS Foundation Trust & Institute of Cancer Research, London, UK. “The uniqueness of this analysis in tracking the timing of ESR1 mutation detection in such a large number of patients will influence the future adoption of this type of assay in the clinic.” According to Turner, the findings from the PADA-1 analysis set the stage for the presentation in the coming months of results from registrational studies that may confirm whether ctDNA-informed treatment approaches can improve outcomes.
Other interesting results at ESMO Breast Cancer 2025 show that ctDNA is also giving further insights into early-stage disease. A retrospective analysis of data from 712 patients in the I-SPY 2 trial, which is investigating individualised treatment strategies based on molecular characteristics (NCT01042379), showed that pre-neoadjuvant therapy (T0) ctDNA levels predicted metastatic recurrence in high-risk early breast cancer (Abstract 5MO). T0 ctDNA levels were significantly higher in patients who later had recurrence than in those who did not. This trend for higher pre-treatment ctDNA level among patients who later had recurrence was seen for HR-positive, HER2-positive and triple-negative breast cancer tumours. In multivariate analysis, T0 ctDNA was the most significant factor in predicting distant recurrence-free survival. “Many previous studies have shown pre-treatment ctDNA detection to be prognostic, but none have been big enough to work out whether it is an independent risk factor or if it is just associated with the aggressiveness of the tumour, such as higher grade or stage,” notes Turner. “This analysis on a large number of patients confirms the substantial independence of ctDNA levels from traditional prognostic factors and validates its role in determining prognosis.”
Programme details
Cabel L, et al. Kinetics and determinants of blood ESR1 mutation under AI and palbociclib in HR+/HER2- metastatic breast cancer patients in the PADA-1 trial. ESMO Breast Cancer 2025, Abstract 1O
Proffered Paper Session 1, 14.05.2025, h. 16:30 – 18:00, Room 14
Magbanua M, et al. Pretreatment circulating tumor (ct)DNA predicts metastatic recurrence in patients (pts) with high-risk early breast cancer (eBC) enrolled in the I-SPY 2 trial. ESMO Breast Cancer 2025, Abstract 5MO
Mini Oral Session 2, 16.05.2025, h. 08:20 – 10:10, Room 1