Final long-term overall survival data from APHINITY confirm the efficacy of dual HER2 blockade in patients with node-positive disease
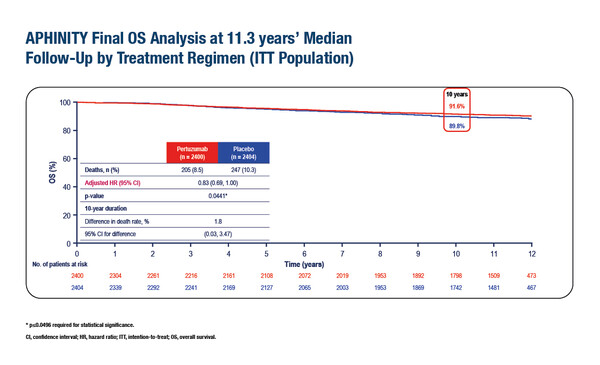
As presented at ESMO Breast Cancer 2025 (Munich, 14–17 May), the final analysis of the phase III APHINITY trial at a median 11.3 years of follow-up confirms an overall survival (OS) benefit when adjuvant pertuzumab is added to trastuzumab plus chemotherapy (hazard ratio [HR] 0.83; 95% confidence interval [CI] 0.69–1.00; p=0.044 [boundary for significance = 0.0496]) in patients with HER2-positive operable breast cancer, with a 10-year OS rate of 91.6% versus 89.8% with placebo (LBA01). Significant improvements in OS were seen with pertuzumab in patients with node-positive (HR 0.79; 95% CI 0.64–0.97) but not node-negative disease (HR 0.99; 95% CI 0.66–1.49), and in those with hormone receptor-positive (HR 0.76; 95% CI 0.60–0.97) but not hormone receptor-negative breast cancer (HR 0.94; 95% CI 0.70–1.26).
In addition, improved invasive disease-free survival (IDFS) was maintained in an updated descriptive analysis at 11.3 years (HR 0.79; 95% CI 0.68–0.92). The 10-year IDFS event-free rates were 87.2% versus 83.8%, respectively. The benefit continued to be clinically meaningful in the node-positive subgroup, while no benefit was seen in the node-negative subgroup. No new cardiac safety concerns emerged.
Prior interim analyses of the APHINITY trial showed significant improvement in the primary endpoint, IDFS, with adjuvant pertuzumab versus placebo added to trastuzumab plus chemotherapy in 4,804 patients with HER2-positive operable breast cancer (N Engl J Med. 2017;377:122–131; J Clin Oncol. 2021;39:1448–1457; J Clin Oncol. 2024;42:3643–3651). Adjuvant pertuzumab with trastuzumab and chemotherapy subsequently became the new standard of care for patients with HER2-positive early breast cancer at high risk of relapse. However, no significant OS benefit was previously reported.
Commenting on the findings, Dr Javier Cortés from the International Breast Cancer Center in Barcelona, Spain, says: “The OS data add considerable weight to the IDFS findings and indicate that where node-negative disease is confirmed, trastuzumab and chemotherapy should remain the adjuvant treatment of choice, but where positive nodes are found, pertuzumab should also be given.” He notes that for patients with node-positive disease who receive upfront surgery without neoadjuvant therapy, these data demonstrate there is still an opportunity to improve survival with adjuvant therapy. He optimistically concludes, “Taken together with response improvements in the neoadjuvant setting, we are doing more, and better, for our patients with HER2-positive early breast cancer, and getting closer to our dream of cure for all.”
Programme details
Loibl S, et al. Adjuvant pertuzumab or placebo + trastuzumab + chemotherapy (P or Pla + T + CT) in patients (pts) with early HER2-positive operable breast cancer in APHINITY: Final analysis at 11.3 years’ median follow-up. ESMO Breast Cancer 2025, LBA1
Proffered Paper Session 2, 15.05.2025, h. 14:00 – 15:30, Room 14