A synergic effect of olaparib, durvalumab and fulvestrant was observed across different mutated subgroups, with higher benefits in BRCA 1 and 2 carriers
In a single-arm phase II trial, the combination of the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib with durvalumab and fulvestrant showed promising activity in patients with ER positive (ER+)/HER2 negative (HER2-) metastatic breast cancer carrying germline BRCA1/2 mutations (gBRCA1/2m), or other somatic or germline pathogenic variants in genes of the homologous recombinational repair (HRR) pathway, or microsatellite instability (MSI) status. Data were presented at ESMO Breast Cancer 2024 (Berlin, 15–17 May) providing new evidence on the synergic effect of PARP inhibitors and immune-checkpoint inhibitors (Abstract 179O).
The study involved 172 patients from 41 centers in France, Spain and Belgium, who had received one prior line of endocrine therapy, including a CDK4/6 inhibitor and ≤ 1 line of chemotherapy for metastatic breast cancer. Next-generation sequencing (NGS) analysis was performed in 165 participants, showing that gBRCA1/2 were the most frequent mutated HRR genes (n=67; 39%) followed by ATM/ATR (16%), FANCA (12%), PALB2 (9%) and CHEK2 (6%). All patients received a combination treatment with olaparib (2 X 300 mg / day ), durvalumab (1500 mg IV / 28 days, started on Day 1 C2), fulvestrant (2 X 250 mg IM Day 1-14-28 and every 28 days), in addition to goserelin for pre-menopausal women and men only.
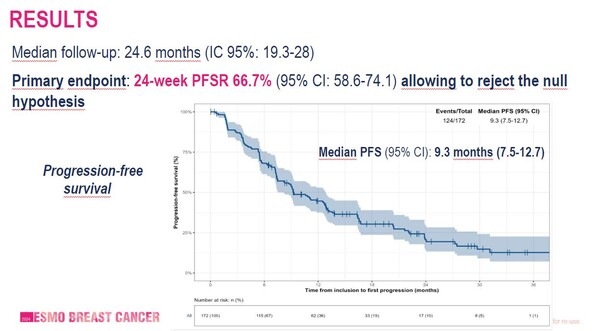
At a median follow-up of 24.6 months, progression-free survival rate at 24 weeks (24w PFSR) – the primary endpoint - was 66% in the 154 evaluable patients (95% CI: 58.2 – 73.6) and 76% in gBRCA1/2 carriers (95%CI: 63.4-86.4). Overall, median progression-free survival (PFS) was 9.3 months (95%CI: 7.5-12.7), objective response rate (ORR) was 41% (95%CI: 33.3-49.0) and median overall survival (OS) was 30 months (95% CI: 26.6-NE) (Figure). A greater effect of combining PARP inhibitor and ICI was observed in the gBRCA1/2m subgroup, where median PFS was 12.6 months, suggesting that that ER signal inhibition remains important for these patients.
No significant differences in efficacy were observed according to previous treatment with a CDK4/6 inhibitor and PD-L1 status.
A manageable safety profile of the triplet therapy was reported, with 33.9% of patients presented at least one treatment-related adverse event of Grade ≥ 3, including anemia (9.4%), asthenia (2.3%), nausea (1.7%) and diarrhea (1.7%). Dose reduction of olaparib was performed in 34.7% of patients and treatment was discontinued in six patients due to adverse events.
Further studies are needed to assess the efficacy of the combination of olaparib, durvalumab and fulvestrant and determine which specific subgroup could benefit most from the triplet.
Abstract discussed:
Guiu S., et al. Combination of olaparib, durvalumab and fulvestrant in patients with advanced ER-positive, HER2-negative breast cancer harboring homologous recombination repair (HRR) deficiency or microsatellite instability (MSI): results of the international phase II DOLAF trial. ESMO Breast Cancer 2024, Abstract 179O
Proffered Paper Session 1, 15.05.2024, h. 16:45 – 17:55, Berlin Hall