Studies provide evidence of potential efficacy for a novel CDK4-selective inhibitor and long-lasting benefits for a pan-AKT inhibitor
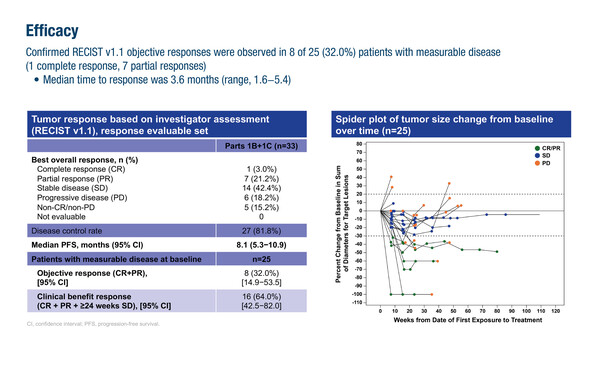
Updated results of the first-in-human study of a novel CDK4-selective inhibitor presented at ESMO Breast Cancer 2024 (Berlin, 15–17 May), revealed promising efficacy and safety in heavily pre-treated patients with hormone receptor (HR)-positive/HER2-negative metastatic breast cancer. The first-in-class next-generation CDK4-selective inhibitor, PF-07220060 plus endocrine therapy (ET; letrozole or fulvestrant) led to 1 complete response (CR) and 7 partial responses (PR) in 25 patients with measurable disease who had progressed on prior CDK4/6 inhibitors plus ET (Abstract 184MO). The clinical benefit response rate was 64.0% and the median progression-free survival (PFS) in the total population was 8.1 months. Responses were irrespective of ESR1 or PI3K pathway mutations.
“The objective response rate and PFS are very promising with PF-07220060 plus ET, considering that all the treated patients had received prior therapy with CDK4/6 inhibitors and about two-thirds had also received fulvestrant and chemotherapy,” says Dr Philippe Aftimos from Institut Jules Bordet – Université Libre de Bruxelles, Brussels, Belgium.
Among the 33 patients, the most frequent treatment-related treatment-emergent adverse events (TEAEs) were neutropenia (grade 3, 18.2%), diarrhoea (grade 3, 0%) and nausea (grade 3, 3.0%). Treatment-related TEAEs led to 1 patient (3.0%) discontinuing treatment and 5 patients (15.2%) had dose reductions. Haematological TEAEs were manageable with dose interruptions or reductions. “It is hypothesised that inhibiting CDK4 alone may reduce haematological toxicity compared with CDK4/6 inhibitors and this seems to be the case with PF-07220060,” comments Aftimos. A phase III study of PF-07220060 plus ET is underway (NCT06105632).
In another presentation, updated results from the phase III CAPItello-291 trial reported that adding the potent selective pan-AKT inhibitor, capivasertib, to fulvestrant in patients with aromatase inhibitor-resistant HR-positive/HER2-negative breast cancer resulted in benefits over placebo that were sustained through time from randomisation to progression on second-line therapy (PFS2), both in the overall (hazard ratio [HR] 0.70) and the PIK3CA/AKT1/PTEN-altered populations (HR 0.52) (Abstract 183MO). The addition of capivasertib also delayed the time from randomisation to the start of subsequent chemotherapy by 4.2 months in the overall population and by 5.0 months in the PIK3CA/AKT1/PTEN-altered populations, with respective HRs of 0.63 and 0.56.
“Based on the CAPItello-291 study, capivasertib, in combination with fulvestrant, recently received approval for the treatment of locally advanced or metastatic breast cancer in patients with one or more PIK3CA/AKT1/PTEN alterations,” says Aftimos, “and these updated results showing a delay to the start of subsequent chemotherapy with capivasertib are highly relevant for patients, since chemotherapy is associated with further adverse events.”
While CDK4/6 inhibitors have become standard-of-care for patients with high-risk disease in the adjuvant setting, Aftimos cautions that alternative treatments are needed for those patients with HR-positive/HER2-negative disease who present with a relapse on or after these agents. “New targeted agents – such as CDK2 and KAT6 inhibitors – in combination with ET are also in development. In addition, the use of antibody–drug conjugates earlier in the treatment sequence – after failure on ET plus CDK4/6 inhibitors or after at least two lines of ET but before chemotherapy – may hold promise and we look to results from studies such as DESTINY-Breast06 investigating trastuzumab deruxtecan in this setting (NCT04494425),” Aftimos concludes.
Abstracts discussed:
Yap TA, et al. First-in-human phase 1/2a study of the first-in-class, next-generation CDK4-selective inhibitor PF-07220060 in combination with endocrine therapy (ET) in patients (pts) with HR+/HER2− metastatic breast cancer (mBC) who progressed on prior CDK4/6 inhibitors (CDK4/6i): safety and efficacy update. ESMO Breast Cancer 2024, Abstract 184MO
Mini Oral Session 2, 17.05.2024, h. 08:30 – 10:05, Hamburg Hall
Rugo HS, et al. Capivasertib and fulvestrant (F) for patients (pts) with aromatase inhibitor (AI)-resistant HR+/HER2– advanced breast cancer (ABC): second progression-free survival (PFS2) and time to first subsequent chemotherapy (TFSC) in the CAPItello-291 trial. ESMO Breast Cancer 2024, Abstract 183MO
Mini Oral Session 1, 16.05.2024, h. 08:30 – 10:00, Munich Hall