Data presentations from DESTINY-Breast04, DESTINY-Breast02 and EMERALD trials confirm the value of trastuzumab deruxtecan and elacestrant in this setting, despite moderate drop-out rates for PROs
At the ESMO Breast Cancer 2023 (Berlin, 11–13 May), three analyses presented refine current knowledge to guide treatment for patients with metastatic breast cancer by providing in-depth safety information and patient reported outcomes (PROs) from recent ground-breaking studies.
Two presentations are reassuring for the antibody–drug conjugate trastuzumab deruxtecan (T-DXd) and highlight no additional safety concerns, either from the physician’s point of view or the patient’s experience.
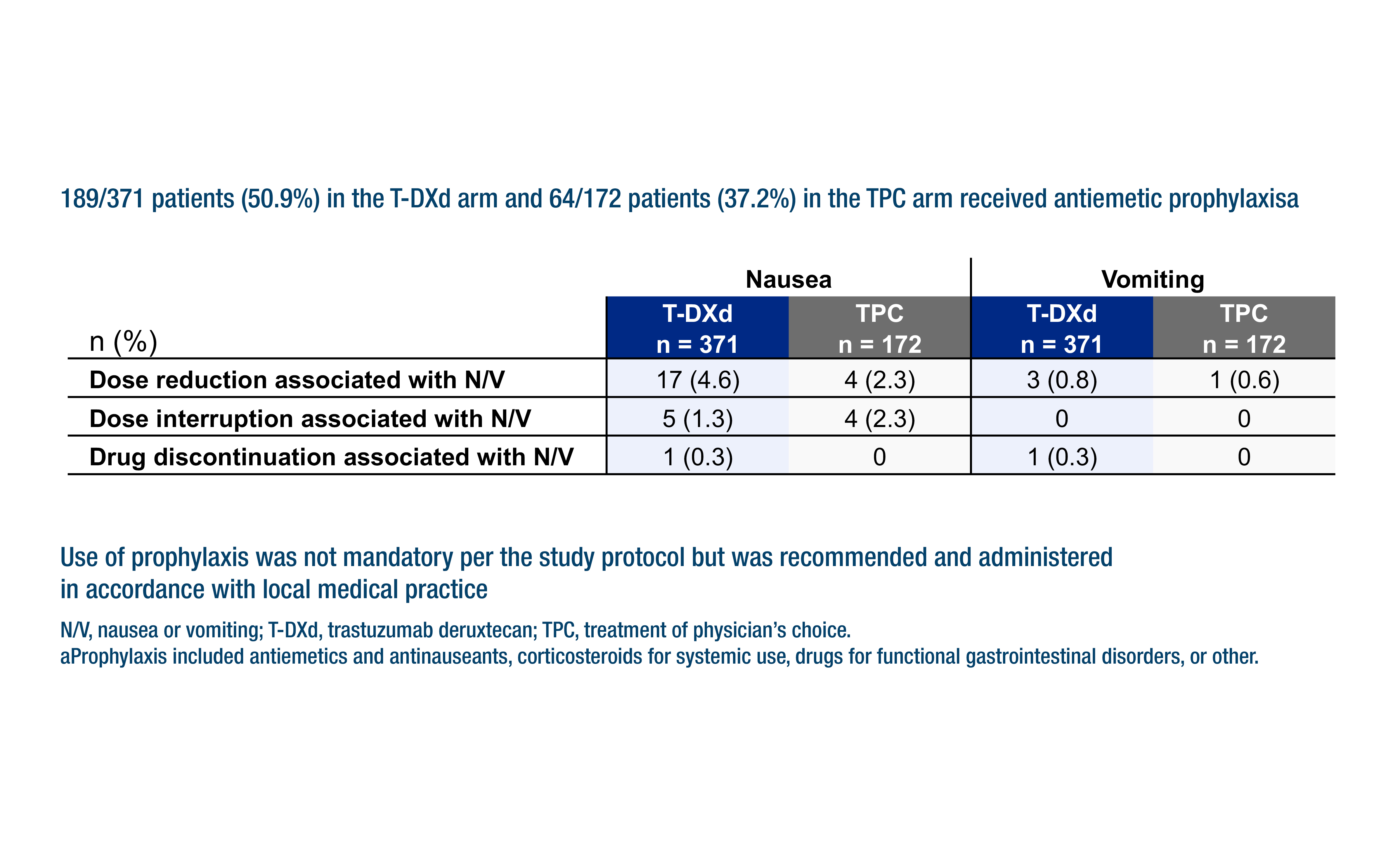
The first was an analysis of the DESTINY-Breast04 trial, which last year confirmed the superior efficacy of T-DXd compared with treatment of physician’s choice (TPC) in previously treated HER2-low unresectable and/or metastatic breast cancer (N Engl J Med. 2022;387:9–20). Additional safety data showed that after a treatment duration of 8.2 months for T-DXd and 3.5 months for TPC, any-grade treatment-emergent adverse events (TEAEs) were lower for T-DXd versus TPC, with exposure-adjusted incidence rates per patient-year of 1.30 and 2.66, respectively (Abstract 185O). In patients receiving T-DXd, the median time to onset and duration of any-grade interstitial lung disease (ILD) were 129 days and 47 days, respectively; six of 13 patients developing drug-related grade 1 ILD were rechallenged following resolution: 1 discontinued due to an adverse event; 2 discontinued due to disease progression and 3 remained on T-DXd.
Any-grade drug-related neutropenia and febrile neutropenia, and the subsequent use of granulocyte colony-stimulating factor, were less common with T-DXd. Conversely, nausea and vomiting were more frequent (79.5% versus 35.5% with TPC), with corresponding rates of anti-emetic prophylaxis of 50.9% and 37.2%. In the T-DXd arm, patients aged ≥65 years had a greater frequency of grade ≥3 TEAEs, or TEAEs that led to treatment discontinuation than patients <65 years.
“It is encouraging to see that although the median treatment duration of T-DXd was more than twice that of TPC, any-grade TEAEs occurred at half the rate,” says Dr Antonio Di Meglio from Gustave Roussy, Villejuif, France. “The effect of adequate nausea and vomiting prophylaxis is also an important take-home message, because this side-effect has a substantial impact on patients’ quality of life (QoL).” In terms of the patients developing grade 1 ILD who were rechallenged, Dr Di Meglio thinks: “Despite the very small number of patients in the study presented, it is reassuring to see that it is possible to safely reintroduce T-DXd upon full resolution of asymptomatic ILD, as recommended by current guidelines. However, ILD remains a significant and potentially life-threatening risk for patients treated with T-DXd, requiring proactive monitoring, diagnosis, and management.”
The second analysis of T-DXd shared PROs data from the phase III DESTINY-Breast02 study, which had already confirmed the superior efficacy of T-DXd over TPC for trastuzumab–emtansine-resistant HER2-positive metastatic breast cancer (Lancet. 2023:April 19. Online ahead of print). In the Congress presentation, the mean change from baseline in EORTC QLQ-C30 global health status (GHS)/QoL remained stable up to cycle 39 for T-DXd and cycle 21 for TPC, with a median treatment duration of 11.3 months for T-DXd and ~4.5 months for TPC. The median time to definitive deterioration (TDD) was longer with T-DXd than with TPC, both for GHS/QoL (14.1 months versus 5.9 months; hazard ratio [HR] 0.56; 95% confidence interval [CI] 0.44–0.71) and all measured QLQ-C30 subscales, including physical functioning (HR 0.46) and pain (HR 0.38), except for nausea/vomiting (HR 1.09) (Abstract 186O). In addition, TDD of the QLQ-BR45 arm symptom subscale was prolonged with T-DXd versus TPC (HR 0.57). Questionnaire compliance was >92% at baseline and >80% at cycles 3–39.
“Raising awareness of the key toxicities of ILD and nausea and vomiting, and their considerable impact on patients’ QoL, will reinforce the message that enhanced patient monitoring may be required to enable effective management to be initiated early. Already, the incidence (including of grade 4 or fatal events) of T-DXd-related ILD appears to be decreasing in more recent DESTINY trials compared with earlier pivotal trials. This could be due to the inclusion of a less heavily treated patient population, as well as to a better understanding of the risk factors involved, better monitoring strategies, and improved, prompt clinical management based on more strict guidelines,” comments Di Meglio.
Finally, a third presentation looked at PROs from the EMERALD trial, which had previously reported the progression-free survival benefits of the selective oestrogen receptor degrader, elacestrant, over standard of care (SoC) in patients with previously treated ER-positive/HER2-negative advanced breast cancer (J Clin Oncol. 2022;40:3246–3256). Data presented at the Congress reported that EORTC QLQ-C30 scores were similar for elacestrant and SoC across all time points for functional and GHS/QoL scales (Abstract 188O). However, elacestrant was associated with a lower rate of very severe nausea (4.0% versus 14.3% by cycle 6) and very severe vomiting (9.1% versus 50.0% by cycle 6) than SoC according to PRO-CTCAE results. There were no clinically meaningful differences between elacestrant and SoC across all time points in adverse events typically observed in patients receiving endocrine therapy for cancer. Elacestrant demonstrated numerically favourable EQ-5D-5L outcomes for mobility, self-care and usual activities. The ratio of PROs tools completed versus PROs tools expected was 80–90% up to cycle 4, dropping to 70% at cycle 6.
Di Meglio comments: “Overall, the PROs data from EMERALD show that QoL was maintained with elacestrant throughout the measurement period. This supports the value of elacestrant as an additional subsequent-line treatment option for ER-positive patients, many of whom have already developed some degree of endocrine resistance.”
While the data presented confirm the role and safety of the investigated agents in metastatic breast cancer, Di Meglio believes that more needs to be done to improve both patients’ compliance in completing their questionnaires and, more broadly, the tools measuring PROs. “The DESTINY-Breast02 and EMERALD analyses both show some reduction in patient compliance with PROs tools over time, and this is reflective of the wider situation in oncology. It is now time to find innovative ways of reducing patient drop out from clinical trials and longitudinal studies. One possible solution could be to implement decentralised and more agile data collection methods, for example by using digital platforms, if we are to get a more complete picture of the patient experience,” he says. “Also, a more tailored approach to measuring PROs might reveal greater insights. In future trials, it may be even more informative to use a tool that is specific to the treatment-associated toxicity of interest, rather than a general health questionnaire, and to look at using different degrees of change from baseline for different domains, instead of one, catch-all value. In this way, we could have a more realistic idea of the patient experience and the value of cancer treatments to inform the research ahead,” Di Meglio concludes.
Abstracts discussed:
Rugo HS, et al. Trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): a detailed safety analysis of the randomized, phase 3 DESTINY-Breast04 trial. ESMO Breast Cancer 2023, Abstract 185O
Proffered Paper Session 1, 11.05.2023, h. 14:00 – 15:30, Hamburg Hall
Fehm TN, et al. Patient-reported outcomes (PROs) from DESTINY-Breast02, a randomized phase 3 study of trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC). ESMO Breast Cancer 2023, Abstract 186O
Proffered Paper Session 1, 11.05.2023, h. 14:00 – 15:30, Hamburg Hall
Cortés J, et al. EMERALD trial analysis of patient-reported outcomes (PROs) in patients with ER+/HER2− advanced or metastatic breast cancer (mBC) comparing oral elacestrant vs standard of care (SoC) endocrine therapy. ESMO Breast Cancer 2023, Abstract 188O
Proffered Paper Session 1, 11.05.2023, h. 14:00 – 15:30, Hamburg Hall