Some studies presented suggest preliminary activity of patritumab–deruxtecan in patients with HR-positive/HER2-negative disease and triple-negative breast cancer
HER3 plays a key oncogenic role in breast cancer, being associated with poor prognosis and resistance to PI3K/AKT/mTOR inhibitors and endocrine therapy. Efforts to target it therapeutically have so far led to unsatisfactory results. Among the novel antibody–drug conjugates (ADCs) that are attracting the interest of researchers, patritumab–deruxtecan (HER3-DXd) shows some promise in targeting HER3, as suggested by a number of presentations at ESMO Breast Cancer 2023 (Berlin, 11–13 May).
One presentation featured data from the single-arm, phase II ICARUS-BREAST01 trial in patients with hormone receptor (HR)-positive, HER2-negative/-low advanced breast cancer, unselected for HER3 expression, progressing on CDK 4/6 inhibitors, any line of targeted/endocrine therapy and one line of chemotherapy (Abstract 189O). The 56 evaluable patients had received a median of two prior systemic therapies for advanced disease and underwent a median of 8 cycles of HER3-DXd. At 3 months, a partial response was observed in 16 patients (28.6%), with stable disease in 30 patients and progressive disease in 10. The most common any-grade adverse events (AEs) were nausea (76.8%) and fatigue (89.3%); fatigue was also the most frequent grade ≥3 AE (14.0%). One patient had grade 1 confirmed interstitial lung disease (ILD).
According to Professor Javier Cortés from the International Breast Cancer Center (IBCC), Barcelona, Spain, this study is important in confirming that patritumab–deruxtecan has preliminary activity in this population of patients, unselected for HER3. “Although only a relatively small number of patients were involved, the 3-month response rate of 28.6%, together with a stable disease rate of 54%, is sufficient to justify moving into randomised trials,” he says. “The safety findings were generally in line with what is expected with this agent, with fatigue and gastrointestinal events being the most common. However, it is interesting that just one patient developed ILD and then only at grade 1. This compares favourably with what we have come to expect with other ADCs.”
The other two presentations concerned the SOLTI TOT-HER3 trial, a window-of-opportunity trial evaluating a single dose of HER3-DXd in patients with treatment-naive HR-positive/HER2-negative early breast cancer.
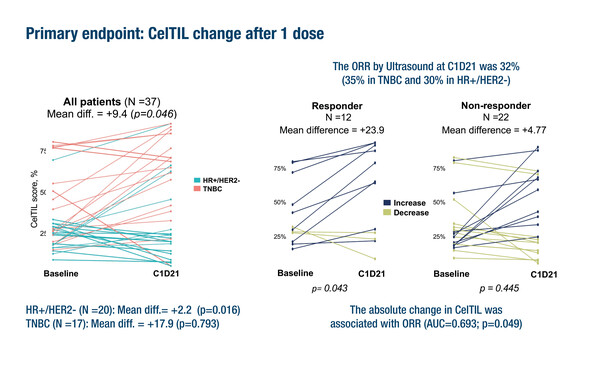
The first presentation reported results from trial part B, involving a HER3-DXd dose of 5.6 mg/kg in 37 evaluable patients (HR-positive/HER2-negative n=20; triple-negative breast cancer [TNBC] n=17) (Abstract 124O). Analysis of baseline and post-treatment paired samples showed a statistically significant change in tumour cellularity and tumour-infiltrating lymphocyte (CelTIL) score overall (p=0.046). The overall response rate (ORR) was 32% (35% in TNBC and 30% in HR-positive/HER2-negative) and was associated with the absolute change in CelTIL (AUC=0.693; p=0.049). There was no association between baseline ERBB3 levels and CelTIL change or ORR. HER3-DXd induced high expression of immune-related genes and suppressed proliferation-related genes. Altogether, 84% of patients reported any-grade adverse events, mainly nausea, fatigue, alopecia and diarrhoea.
“The activity data are extremely provocative, with a promising response rate after just one dose of HER3-DXd. And it is good to see preliminary activity in TNBC, although more data are required to confirm this,” says Cortés. However, he is more cautious in his interpretation of the CelTIL findings. “We see in this trial that change in CelTIL score was greater in patients with TNBC compared with HR-positive patients. It could be that this measure, which was developed in HER2-positive patients, is not the most appropriate endpoint for patients with HR-positive/HER2-negative breast cancer,” he says.
The second presentation from the SOLTI TOT-HER3 trial involved data from a correlative analysis of part A, looking at the association between early response to HER3-DXd (6.4 mg/kg) and HER2 levels (Abstract 3MO). HER2 status by immunohistochemistry (IHC) among the 77 patients included was 0 in 32%, 1+ in 38% and 2+ in 30%. High CelTIL response was most common among patients with HER2 1+ (45%) and HER2 0 (40%), with only 13% being seen in patients with HER2 2+ (p=0.034). A significant association was seen between CelTIL response and high ERBB2 mRNA (odds ratio [OR] 0.34; p<0.001; AUC=0.71), which was independent of HER2 IHC expression (p=0.002), PAM50 subtype (p=0.001) or proliferation (p=0.003). A significant association was also seen between CelTIL response and RNA-based HER2 amplicon signatures (OR 0.20; p=0.005; AUC=0.74). A high HER2 copy number signal was associated with a low CelTIL response (p=0.005).
“The data from this analysis show that low HER2 expression, mRNA or copy number in patients with HR-positive/HER2-negative breast cancer is associated with a higher response to HER3-DXd,” comments Cortés. “However, the significance of this finding requires clarification. In general, HER2 status is reflective of HR status, such that the lower the HER2 expression, the lower the HR expression. It would be useful, therefore, to see if the CelTIL response also correlates with expression of hormone receptors,” he says.
Abstracts discussed:
Pistilli B, et al. A phase 2 study of patritumab deruxtecan (HER3-DXd), in patients (pts) with advanced breast cancer (ABC), with biomarker analysis to characterize response to therapy (ICARUS-BREAST01). ESMO Breast Cancer 2023, Abstract 189O
Proffered Paper Session 2: Best abstracts, 12.05.2023, h. 16:45 – 18:15, Berlin Hall
Oliveira AMA, et al. Patritumab deruxtecan (HER3-DXd) in hormonal receptor-positive/HER2-negative (HR+/HER2-) and triple negative breast cancer (TNBC): results of part B of SOLTI TOT-HER3 window of opportunity trial. ESMO Breast Cancer 2023, Abstract 124O
Proffered Paper Session 2: Best abstracts, 12.05.2023, h. 16:45 – 18:15, Berlin Hall
Brasó-Maristany F, et al. HER2 expression and early response to patritumab deruxtecan (HER3-DXd) in early-stage hormone receptor-positive/HER2-negative (HR+/HER2-) breast cancer (BC): a correlative analysis from SOLTI-TOT-HER3 trial. ESMO Breast Cancer 2023, Abstract 3MO
Mini Oral Session 2, 12.05.2023, h. 08:45 – 10:15, Berlin Hall