In the SASCIA trial, sacituzumab govitecan was associated with a higher incidence of adverse events and dose delays than treatment of physician’s choice in patients with HER2-negative breast cancer at high risk of relapse, but dose reductions occurred with a similar frequency in both arms
The phase III SASCIA trial is currently investigating whether the antibody–drug conjugate (ADC) sacituzumab govitecan may be effective in patients with HER2-negative breast cancer with residual disease after standard neoadjuvant chemotherapy (NACT) (NCT04595565). Results of a pre-planned safety interim analysis, presented at ESMO Breast Cancer 2022, indicate that the safety profile of sacituzumab govitecan was manageable (Abstract 58O).
In April 2020, sacituzumab govitecan was granted accelerated US FDA approval as a treatment for triple-negative breast cancer (TNBC) after at least two prior therapies for metastatic disease, and it has recently received fast-track designation for non-small-cell lung cancer and urothelial carcinoma. “Sacituzumab govitecan may be considered transformative in the setting of metastatic TNBC, as it halves the risk of death from a disease with limited therapeutic options in advanced lines of treatment,” says Dr Carmen Criscitiello, European Institute of Oncology (IEO), Milan, Italy. Due to its demonstrated efficacy, the agent is now investigated in other settings.
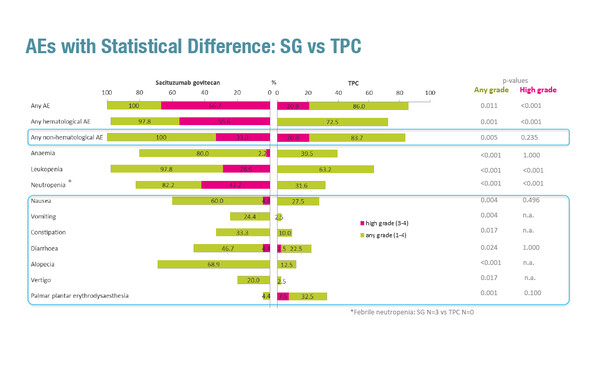
In the interim analysis of the SASCIA trial, among 88 patients with HER2-negative breast cancer at high relapse risk after neoadjuvant treatment, grade 1–4 adverse events (AEs) occurred in all 45 (100%) patients receiving sacituzumab govitecan and in 37 of 43 (86.0%) patients randomised to receive treatment of physician’s choice (TPC: capecitabine [n=32] or observation [n=11]). Corresponding rates for grade 3–4 AEs were 66.7% and 20.9%. All-grade and grade 3–4 haematological AEs generally occurred more frequently with sacituzumab govitecan compared with TPC. For non-haematological toxicities, all-grade AEs, including nausea, vomiting, constipation, diarrhoea and alopecia, were significantly more common with sacituzumab govitecan than with TPC. There were no deaths.
Six patients receiving sacituzumab govitecan and three receiving capecitabine discontinued treatment prematurely. Dose delays were more frequent with sacituzumab govitecan. At least one dose delay was required by 30 patients (66.7%) receiving sacituzumab govitecan (46.7% due to haematological AEs and 6.7% due to non-haematological AEs) compared with 13 (43.3%) patients receiving capecitabine (10.0% due to haematological AEs and 23.3% due to non-haematological AEs). At least one dose reduction was required by 12 patients (26.7%) receiving sacituzumab govitecan (13.3% due to haematological AEs and 11.1% due to non-haematological AEs) and by 9 (28.1%) patients receiving capecitabine (3.1% due to haematological AEs and 18.8% due to non-haematological AEs).
"ADCs have already demonstrated the ability to change the course of the disease in all subgroups of breast cancer, exhibiting an improvement in overall survival across the breast cancer spectrum. And ADCs are quickly moving into earlier lines of treatment,” concludes Criscitiello. “However, their role is not confined to the metastatic setting; they will very likely have a role in the early breast cancer setting – ongoing trials aim to demonstrate if they will fulfil the promise.”
The SASCIA trial involves patients with HER2-negative breast cancer and residual disease after NACT or hormone receptor-positive disease with a clinical and post-treatment pathological stage (CPS) + oestrogen receptor status and grade (EG) score ≥3 or 2 and residual lymph node disease after NACT. At baseline, Ki67 >20% was more common in the sacituzumab govitecan arm (64.4%) compared with the TPC arm (48.8%). The pre-planned safety interim analysis was performed after the first 50 randomised patients had completed 4 cycles of therapy. The SASCIA trial is expected to enroll around 1,200 participants and primary completion is estimated for December 2026.
Abstract discussed:
Marmé F, et al. Safety interim analysis (SIA) of the phase III postneoadjuvant SASCIA study evaluating sacituzumab govitecan (SG) in patients with primary HER2-negative breast cancer (BC) at high relapse risk after neoadjuvant treatment. ESMO Breast Cancer 2022, Abstract 58O
Proffered Paper Session 2, 04.05.2022, h. 16:45 – 18:15, Berlin Hall
Watch the session also on the Congress virtual platform.