The Pan-Asian adaptation of the ESMO guidelines introduces some changes to the standard of care in Europe, including diagnostic tests and the use of cytoreductive nephrectomy
In the second of three presentations on Pan-Asian adapted ESMO Clinical Practice Guidelines (PAGA) during the ESMO Asia Virtual Oncology Week 2021, Prof. Ravindran Kanesvaran, National Cancer Centre Singapore, Singapore, outlines the five key changes made to the 2019 ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of renal cell carcinoma (Ann Oncol. 2019;30:706–720).
One change considers the specific laboratory tests recommended to confirm a suspicion of renal cell carcinoma (RCC). “Based on acceptability and applicability, it was felt that the Pan-Asian guidelines should specify which tests are considered essential – full blood count and renal profile tests – and that the remaining tests may be carried out to facilitate diagnosis/prognosis,” says Kanesvaran.
Another change concerns the ESMO guideline’s recommendation for a kidney-lesion biopsy prior to taking a decision about nephrectomy for a patient with local/locoregional disease. “In some Asian countries, renal biopsy is sometimes omitted, particularly if imaging techniques show typical features of clear cell carcinoma and nephrectomy is scheduled, and this is reflected in the Pan-Asian adapted guidelines text,” explains Kanesvaran.
An area that Kanesvaran says attracted quite a lot of controversy among the Asian panel involved in the development of the PAGA on renal cell carcinoma focuses on the role of cytoreductive nephrectomy (CN) in the management of advanced/metastatic disease. “Since the publication of the phase III CARMENA study (N Engl J Med. 2018;379:417–427), CN has not been regarded as standard of care in Europe for the treatment of patients with intermediate- or poor-risk metastatic RCC requiring systemic treatment,” says Kanesvaran. “Similarly, it is current practice in some Asian centres to only perform CN if the patient is fit enough to tolerate the procedure, has a low metastatic burden or if a reasonable level of debulking and symptom relief can be achieved – this point has now been made in the adapted guidelines,” he says.
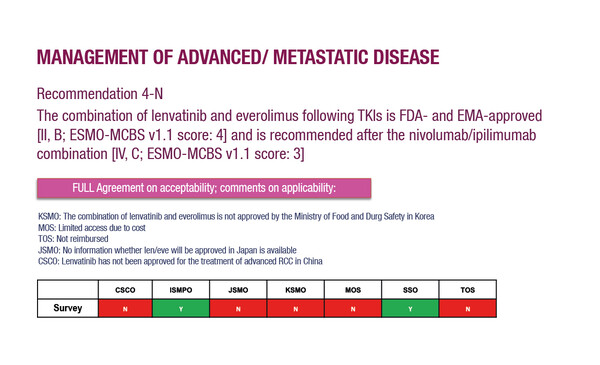
The other main changes in the PAGA on RCC include the removal of recommended radiotherapy (RT) techniques for the management of advanced/metastatic disease, reflecting the fact that RT is not generally used in Asia in the treatment of unresectable primary tumours owing to a lack of therapeutic benefit, but is used mostly in the palliative setting. There was also recognition among the Asian experts that lenvatinib plus everolimus is still a good option for treatment in the second-line setting.
For many Asian countries, de novo development of guidelines is not feasible because of a lack of time, expertise and resources, so international guidelines, like those provided by ESMO, play a key role in influencing treatment decisions. Despite this, variations in healthcare systems, along with differences in access to treatments and economics across countries in Asia, may be an obstacle to the widespread uptake of the guidelines. The PAGA can help in overcoming some of these limitations. “The experts involved in the adaptations represent the mixture of healthcare systems in the region, ensuring that Pan-Asian adapted recommendations fully reflect local needs,” says Kanesvaran. Two tables in the PAGA publication will be particularly important in this respect: one details which medicines are available in which Asian countries, and the other shows the ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scores for new therapies in RCC. “The information in these tables is important when considering value-based care for patients,” he concludes. “It can be used in negotiations between healthcare agencies and pharmaceutical companies regarding reimbursement issues, and it will play an important role in pressing for public healthcare policy changes.”
The PAGA project was initiated in 2016 with the aim of tailoring ESMO Clinical Practice Guideline recommendations to ensure that they incorporate ethnic, scientific, socioeconomic and local practice elements to meet the needs of cancer patients in Asia. Building on successful collaborations with key opinion leaders from seven partner oncology societies in China, India, Japan, South Korea, Malaysia, Singapore and Taiwan, ESMO is now expanding the number of partners to ten, with the addition of societies from the Philippines, Indonesia and Thailand. On occasion of the ESMO Asia Virtual Oncology Week 2021, a dedicated web page has been launched where users can find all Pan-Asian Guidelines Adaptations produced so far in one single place.
Kanesvaran R. RCC: Presentation of the Pan-Asian adapted ESMO Clinical Practice Guideline recommendations. ESMO Asia Virtual Oncology Week 2021
Pan-Asian Adapted ESMO Clinical Practice Guidelines lecture on renal cell carcinoma (RCC). 21.11.21, h. 15:15 – 15:35 (Singapore time), Channel 1