Novel approaches including the use of a JAK-STAT inhibitor, a nomogram and assessment of eosinophil proportions are presented for improving the management of immune-related adverse events
Results from three real-world studies presented at the ESMO Asia Congress 2023 (Singapore, 1–3 December) may help to shape the more effective management of immune-related adverse events (irAEs) by providing some practice-relevant evidence on treatment and prediction approaches.
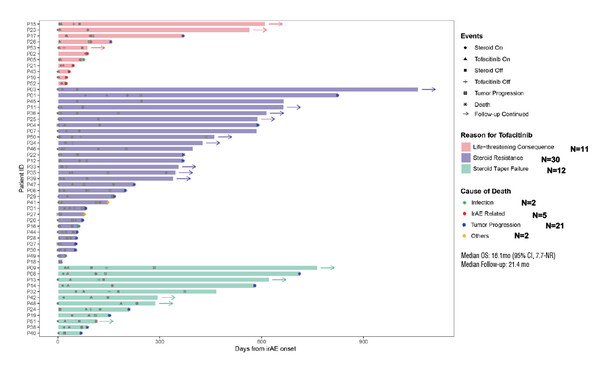
One presentation assessed the JAK-STAT inhibitor tofacitinib for the management of difficult-to-treat irAEs (Abstract 433MO). Involving 53 patients, the median time from irAE onset to tofacitinib therapy was 17 (range, 2–186) days and median duration of tofacitinib treatment was 48.5 (range, 3–277) days. The remission rate was 54.5% in 11 patients with life-threatening irAEs, 96.7% in 30 patients with steroid-resistant irAEs and 100% in 12 patients with steroid taper failure.
“The findings with tofacitinib are particularly interesting, as we are constantly looking for agents that have a more targeted action than steroids and therefore can be expected to have a lower impact on immune response to cancer,” says Dr Marco Donia from Copenhagen University Hospital, Herlev-Gentofte, Denmark, commenting on the data presented. “The high rates of irAE remission seen in patients with steroid resistance were encouraging and it is expected that agents such as tofacitinib may eventually migrate from steroid-resistant irAEs to earlier stages of management. Steroids are highly unselective immunosuppressive agents and, in addition, these agents have important side effects and may jeopardise the immune response to cancer. I agree that the real-world evidence presented, coupled with previous case reports, warrants prospective studies on tofacitinib.”
Two other studies reported on novel strategies for predicting the likelihood of irAE development. In one of these studies, a prediction nomogram for irAEs – based on a combination of clinical characteristics and laboratory data (PD-L1 status, lymphocyte level, alanine transaminase level, treatment duration, free T4 and plasma Epstein-Barr virus DNA titre) – was tested and validated in 336 patients with recurrent or metastatic nasopharyngeal carcinoma (NPC) receiving the PD-1 inhibitor, toripalimab (Abstract 356O). Patients identified as being high-risk for irAEs had a significantly better overall and progression-free survival than those with a low risk of irAE development (p<0.05). According to Donia, it will now be interesting to see if this nomogram can be developed further and to observe if the promising results reported in the setting of NPC can be extended to other tumour types and other checkpoint inhibitors.
Finally, a retrospective analysis examined whether eosinophil proportions could be used as a biomarker for predicting irAEs in 614 patients receiving checkpoint inhibitor therapy for various cancers (Abstract 434MO). Mean eosinophil proportions prior to the second course of therapy were higher in patients developing irAEs compared with those not developing irAEs (p=0.05), regardless of ICI type (anti-PD-1, anti-CTLA-4 plus anti-PD-1, or anti-PD-L1 therapy). The relationship was found across oesophageal, melanoma, gastric, head and neck, lung, renal cell and urothelial carcinomas. “This relatively simple approach of measuring eosinophils after treatment initiation may provide an effective way to stratify patients at a high risk of irAEs requiring a more intensive follow up, and could be an especially useful approach for healthcare systems with constrained resources,” highlights Donia.
He concludes, “As immunotherapy continues to grow and expand into different tumour types, there is an increasing need to gain a better understanding of immune-related toxicities, their relationship to outcomes, risk factors for development and appropriate treatment strategies beyond steroids. It is now commonly accepted that clinical trials have some limitations in the information they provide, and real-world data are increasingly helping to fill gaps in knowledge.”
Abstracts discussed:
Liu Q, et al. Tofacitinib for the treatment of immune-related adverse events in cancer immunotherapy: A multi-center study. ESMO Asia Congress 2023, Abstract 433MO
Mini Oral Session: Supportive and Palliative Care, 02.12.2023, h. 14:30 – 16:00 SGT, Hall 402
Chen QY, et al. Construction of an immune-related adverse events prediction model for teripulimab in the treatment of recurrent metastatic nasopharyngeal carcinoma and its analysis with survival prognosis. ESMO Asia Congress 2023, Abstract 356O
Proffered Paper Session: Head and Neck Cancer, 02.12.2023, h. 09:00 – 10:20 SGT, Hall 404
Tasaki Y, et al. Increased eosinophil is a universal biomarker for immune-related adverse events induced by immune checkpoint inhibitors in various cancer patients: A retrospective multidisciplinary study. ESMO Asia Congress 2023, Abstract 434MO
Mini Oral Session: Supportive and Palliative Care, 02.12.2023, h. 14:30 – 16:00 SGT, Hall 402