Inequities in access to targeted cancer treatments are due to the high costs of tests, limited number of approved medicines or clinical trials and poor communication with patients, as two new surveys report
Molecular testing is now becoming critical in a growing number of cancer types, leading to better outcomes in cancer patients. However, this is only true in regions where there is access to appropriate molecular testing and the relevant targeted treatments.
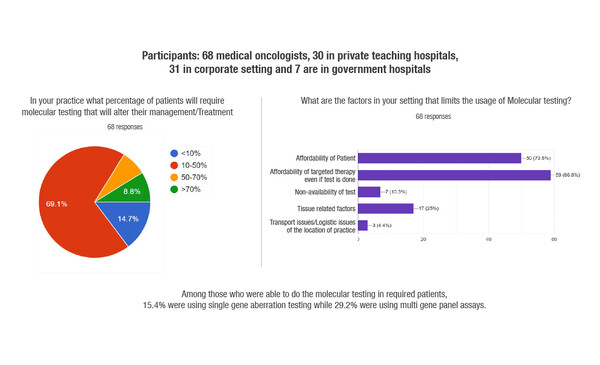
According to the results of a survey conducted among 68 medical oncologists in India, and presented at the Molecular Analysis for Precision Oncology Virtual Congress 2021 (MAP 2021 Virtual), budget constraints and medicine availability are still major obstacles to the widespread use of precision medicine in the country (Abstract 67P). According to survey results, the main barriers to accessing these tests were considered to be the cost of the molecular test (73.5%) and the cost of the targeted treatment (86.8%): 82–84% of respondents thought that more than half of their patients would not be able to afford either the molecular test or the associated targeted treatment. Findings show that 69% of respondents said that 10–50% of their patients needed a molecular test that would alter their treatment.
These results mirror previous findings from surveys and questionnaires highlighting global variations in the uptake of molecular testing, with generally lower uptake in some regions such as Latin America, and the rest of the world, including China, Saudi Arabia and India, compared with North America, Europe and parts of Asia (J Thorac Oncol 2020;15:1434-1448; PLoS One 2018;13:e0202865; JCO Oncol Pract 2020;16:e770-e778).
In many countries, the public healthcare system does not support even minimal testing for standard-of-care therapies and issues with quality are common.
“Unfortunately, the more precision medicine advances, the more disparity we see. Our experience with clinicians in Latin America highlights the reality that the use of molecular testing right now is very dependent on the healthcare budgets in each country, support programmes from pharmaceutical companies and patient out-of-pocket expenses” says Dr Rodrigo Dienstmann from Grupo Oncoclinicas, São Paulo, Brazil.
“In many countries, the public healthcare system does not support even minimal testing for standard-of-care therapies. In addition, while we know that high quality is critical for accurate diagnosis, issues with quality are common. Failure rates, particularly in relation to sample preparation, are high and this has led to clinicians losing confidence in the results of the tests.” The study presented at MAP 2021 Virtual also highlights possibly one of the most important barriers to molecular testing: availability of the appropriate targeted therapy. “If the test yields a positive result, the clinician needs to fight for access to the relevant treatment,” explains Dienstmann. “In developing countries, or those with financial constraints, such therapies are often not available through public healthcare systems or clinical trials, so clinicians are faced with being unable to provide their patient with the optimal treatment. The result is that, although recognising the importance of comprehensive molecular testing, some clinicians may choose not to subject their patients to something they are unlikely to benefit from.”
Patient understanding of biomarker analysis – the implications of testing on treatment choices, which can improve outcomes and quality of life – is an important aspect of molecular diagnosis. Presented at MAP 2021 Virtual, a survey of 128 patients and caregivers in Canada revealed that more than two-thirds of respondents (69.1%) were unaware that biomarkers could help to determine the best treatment for them (Abstract 57P). Only 16% said their physician had explained about testing prior to treatment. Among patients undergoing biomarker testing, targeted therapy or immunotherapy was received by none prior to testing compared with 23.5% after testing. The most common obstacles to biomarker testing were lack of awareness (20.6%) and lack of availability (14.7%).
“We know that patients need to be engaged in the testing process, but this survey highlights the fact that most patients have no understanding of the implications of testing,” comments Dienstmann, highlighting that clinicians need to better communicate with, and educate patients to this regard. “And this includes explaining both the risks and the benefits of such testing,” he adds. “Molecular testing is now required for standard-of-care therapy for many different tumour types. However, we have moved on from the situation of ‘one mutation, one drug’. With advances in next-generation sequencing (NGS) and comprehensive profiling, testing may reveal actionable alterations for which there are no currently approved treatments – opening the door to off-label therapy use or enrolment in a clinical trial – or alterations that may have implications for germline testing in the patient and family members linked to a suspicious hereditary cancer. These are complex issues which, however, need to be explained to patients in order to make shared decisions.”
Abstracts presented at MAP 2021 Virtual:
Koyyala VPB et al. Challenges to Molecular testing for selecting targeted therapies for oncology patients in India. MAP 2021 Virtual, Abstract 67P
El Bizri M. Patients’ and caregivers’ perspective on biomarker testing across Canada. MAP 2021 Virtual, Abstract 57P.