Sustained benefit of PARPi treatment may help to put the recent concerns on how and when to use these agents in women into context
PARP inhibitor (PARPi) therapy has transformed the care of women with recurrent ovarian cancer, but recent long-term sub-group analyses yielded overall survival (OS) data that raised concerns regarding the use of PARPi in tumours with certain molecular profiles. “The removal last year of US FDA approval for a number of agents, including maintenance niraparib for non-BRCA-mutated recurrent ovarian cancer, has generated some uncertainty among clinicians globally about the efficacy of PARPi in this setting and others, with clinical implications of a potential lack of confidence for patient treatments,” says Dr Susana Banerjee from The Royal Marsden NHS Foundation Trust, The Institute of Cancer Research, London, UK, and Scientific Co-Chair of the ESMO Gynaecological Cancers Congress 2023 (Barcelona, 23–24 February). According to her, encouraging long-term data from three phase III studies in patients with ovarian cancer presented at the Congress indicate sustained benefit of PARPi treatment and may help to put the recent OS analyses into context.
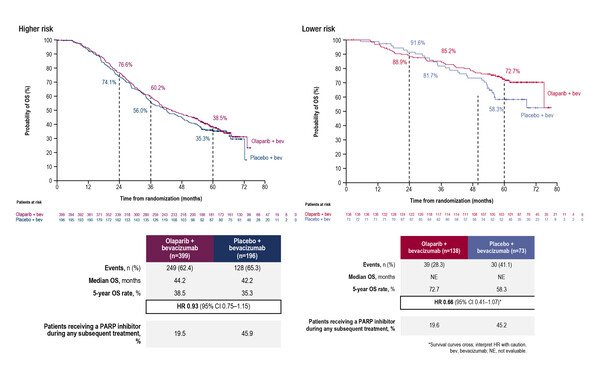
A post hoc analysis of the PAOLA1/ENGOT-ov25 trial explored 5-year OS according to clinical risk and homologous recombination deficiency (HRD) status in patients receiving maintenance bevacizumab with olaparib or placebo (Abstract 32O). Clinical risk was classified as higher-risk disease (stage III disease with upfront surgery and residual disease or neoadjuvant chemotherapy or stage IV disease) and lower-risk (stage III disease with upfront surgery and no residual disease). The addition of olaparib to bevacizumab improved OS rate in both higher risk (38.5% versus 35.3%) and lower risk (72.7% versus 58.3%) patients compared with placebo, an effect that was evident in patients with HRD-positive disease and BRCA-mutated disease. Reflecting progression-free survival (PFS) results seen in the overall trial population (N Engl J Med 2019;381:2416–2428), an OS benefit was not observed in patients with HRD-negative disease.
The two other studies discussed at the meeting looked at the use of niraparib. An ad hoc interim OS analysis of the NORA study, the Chinese partner study to the NOVA study, showed a trend for a favourable OS outcome with niraparib versus placebo in the intention-to-treat (ITT) population (hazard ratio [HR] 0.692; 95% confidence intervals [CI] 0.447–1.072) (Abstract 35O). Similar findings were made in the germline BRCA-mutated population (HR 0.882; 95% CI 0.391–1.990) and the non-germline BRCA-mutated population (HR 0.624; 95% CI 0.368–1.056). It should be noted that the results were adjusted for subsequent PARPi use in the placebo arm. A long-term analysis of the PRIMA/ENGOT-OV26/GOG-3012 study investigated maintenance niraparib in patients with newly diagnosed advanced ovarian cancer at high risk of relapse following response to platinum (Abstract 33O). Estimated 4-year PFS rates for niraparib and placebo were 38% and 17%, respectively, in the HRD-positive population and 24% and 14%, respectively, in the ITT population. Patients free from death or progression at 2 years had a high probability of remaining progression-free or alive at 4 years.
“These studies give some indication of the long-term efficacy of niraparib but one of the most immediate clinical questions we now need to answer for niraparib is: what is the magnitude of clinical benefit among patients with HRD-negative tumours?,” says Banerjee. Although niraparib has European regulatory approval independent of biomarker status, use in these patients should be justified by sustained, clinically relevant benefit.”
Moving forward, refinement of PARPi use will rely heavily on more specifically defining the patients with ovarian cancer most likely to benefit. “There is no doubt that patients with BRCA mutation should continue to be offered maintenance PARPi. However, treatment decisions based on HRD status will be better informed by the use of more sensitive testing techniques, which may also include the integration of other clinical and biological factors,” says Banerjee. “We also need to develop strategies that will allow us to optimise long-term efficacy while minimising the development of serious side-effects, such as myelodysplastic syndrome and acute myeloid leukaemia.” A changing role for PARPi as maintenance therapy may be on the horizon. “As the numbers of patients receiving PARPi in the first-line setting expand, so the space for maintenance PARPi in the recurrent setting will become smaller,” Banerjee explains. “Antibody–drug conjugates may be a useful alternative to PARPi in this setting and several phase III trials – including the GLORIOSA (NCT05445778) and UP-NEXT (NCT05329545) trials – are currently underway.”
Abstracts discussed:
Lorusso D, et al. 5-year (y) overall survival (OS) with maintenance olaparib (ola) plus bevacizumab (bev) by clinical risk in patients (pts) with newly diagnosed advanced ovarian cancer (AOC) in the phase III PAOLA-1/ENGOT-ov25 trial. ESMO Gynaecological Cancers Congress 2023, Abstract 32O
Proffered Paper Session, 23.02.2023, h. 11:45 – 12:45, Auditorium 113
Wu X, et al. Overall survival of niraparib with individualized starting dose as maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer adjusted for subsequent PARPi use in placebo group: results from an ad hoc interim analysis for the phase 3 NORA study. ESMO Gynaecological Cancers Congress 2023, Abstract 35O
Proffered Paper Session, 23.02.2023, h. 11:45 – 12:45, Auditorium 113
Gonzalez Martin AJ, et al. PRIMA/ENGOT-OV26/GOG-3012 study: long-term conditional PFS. ESMO Gynaecological Cancers Congress 2023, Abstract 33O
Proffered Paper Session, 23.02.2023, h. 11:45 – 12:45, Auditorium 113