Progression-free survival was longer with tumour-specific versus standard chemotherapy in two trials, but further work is needed to improve prognosis for these patients
Encouraging data from studies relating to precision medicine for cancers of unknown primary (CUPs) were presented at the ESMO Congress 2023 (Madrid, 20–24 October). CUPs have a poor prognosis due to a lack of standardised work-up aimed at pinpointing the primary tumour site, and ESMO Clinical Practice Guidelines highlight the need for new data to support techniques such as next-generation sequencing to identify potential therapeutic targets (Ann Oncol. 2023;34:228–246).
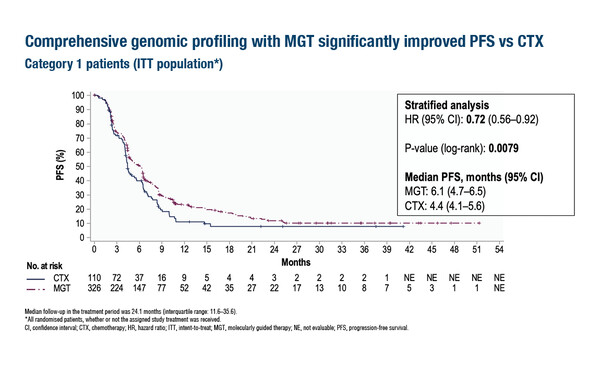
Data from the phase II CUPISCO trial (LBA16) showed significantly improved median progression-free survival (PFS) – the primary endpoint of the study – in patients who received molecularly-guided therapy (MGT; 6.1 months) versus standard platinum-based chemotherapy (4.4 months), with a hazard ratio (HR) of 0.72 (95% confidence interval [CI] 0.56–0.92; p=0.0079). Overall survival (OS) data were immature but tended towards improvement with MGT (HR 0.82; 95% CI 0.62–1.09; p=0.1779). Objective response rates were 17.8% with MGT and 8.2% with chemotherapy, with similar durations of response (HR 0.95; 95% CI 0.33–2.72). Adverse event rates were generally similar or lower with MGT versus chemotherapy and there was no difference between treatment arms for quality-of-life measures.
In CUPISCO, patients with newly diagnosed, unfavourable, non-squamous CUP received 3 induction chemotherapy cycles, then those with disease control were randomised 3:1 to MGT (n=326; investigator’s choice after molecular tumour board advice) or ≥3 more cycles of platinum-based chemotherapy (n=110).
Prof. Olivier Michielin from Geneva University Hospitals, Switzerland, found the study data encouraging, adding that, “This is a positive trial for CUPs, which adds to the body of evidence for a precision approach in a patient population whose standard of care is unsatisfactory. However, we know that patients with CUPs generally have a poor prognosis and including only patients with disease control after 3 cycles of chemotherapy in the trial might select more favourable patient cases. This should be kept in mind when interpreting the results. Whether this trial is practice changing depends on location – patients may already be referred for genome profiling and precision oncology programs in some centres, but this is not always widely available.”
In a phase III trial presented at the Congress, a PFS benefit was also observed in 182 previously untreated patients with CUP site-specific therapy guided by a 90-gene expression assay versus empiric chemotherapy (Abstract 1208MO). Median PFS was 9.6 months with site-specific therapy versus 6.6 months with empiric chemotherapy (HR 0.68; 95% CI 0.49–0.93; p=0.017) at a median follow-up of 42.9 months. Median OS was longer with site-specific therapy than with empiric therapy (28.2 months versus 19.0 months, respectively; HR 0.74; 95% CI 0.52–1.06; p=0.098) and grade ≥3 adverse events were similar for both arms. Michielin comments, “This is an interesting trial, further showing the importance of identifying the primary tumour site to allow the use of organ-specific chemotherapy regimens. However, as seen in the CUPISCO trial, survival rates were still poor, highlighting that considerable unmet needs remain.”
Regarding diagnosis, data presented from the CUP-ONE study of 418 samples showed similar accuracy when using the CancerTYPE ID (CTID) 92-gene expression signature data versus specialist centralised immunohistochemistry (C-IHC; 11 markers) compared with a multidisciplinary team-determined reference diagnosis, although CTID showed greater accuracy for classifying cholangiocarcinoma (LBA100). Michielin notes, “The performance of CTID appears to be similar to C-IHC, except in cholangiocarcinoma, a relatively rare tumour type. Importantly, defining the gold standard reference diagnosis itself remains a challenge in such studies, but we need to continue to develop tools to improve the accuracy of diagnosis.”
Reflecting on the overall changing landscape of CUPs management, Michielin says, “Genomic profiling is becoming more frequent due to the increased presence of precision oncology programmes and molecular tumour boards. Ongoing trials in CUPs that include tissue and blood profiling may enable new therapeutic strategies, including targeted and immunotherapies, to help further improve outcomes.”
Abstracts discussed:
Mileshkin L, et al. Primary analysis of efficacy and safety in the CUPISCO trial: A randomised, global study of targeted therapy or cancer immunotherapy guided by comprehensive genomic profiling (CGP) vs platinum-based chemotherapy (CTX) in newly diagnosed, unfavourable cancer of unknown primary (CUP). ESMO Congress 2023, LBA16
Proffered Paper Session 1 – Basic science and translational research, 21.10.2023, h. 10:15 – 11:45, Salamanca Auditorium – Hall 3
Luo Z, et al. A randomized phase III trial of site-specific therapy guided by the 90-gene expression assay versus empiric chemotherapy in patients with cancer of unknown primary. ESMO Congress 2023, Abstract 1208MO
Mini Oral Session – Basic science & translational research, 22.10.2023, h. 08:30 – 10:00, Santander Auditorium – Hall 9
Wasan HS, et al. CUP-ONE Trial: A prospective double-blind validation of molecular classifiers in the diagnosis of Cancer of Unknown Primary and clinical outcomes. ESMO Congress 2023, LBA100
Mini Oral Session – Basic science & translational research, 22.10.2023, h. 08:30 – 10:00, Santander Auditorium – Hall 9