Technological and scientific advances over the last few decades have shifted the focus of cancer treatment towards precision medicine. The most successful approaches usually involve targeting an initial, often clonal, truncal genetic event, rather than mutations occurring later in a tumour’s evolution – referred to as branch mutations – that often create specific subclones. Targeting the initiating truncal event is advantageous because every tumour cell should carry that initiating mutation. Reversing or negating the initiating mutation may reveal the deleterious effects of the later mutations that otherwise confer an advantage to the tumour cell.
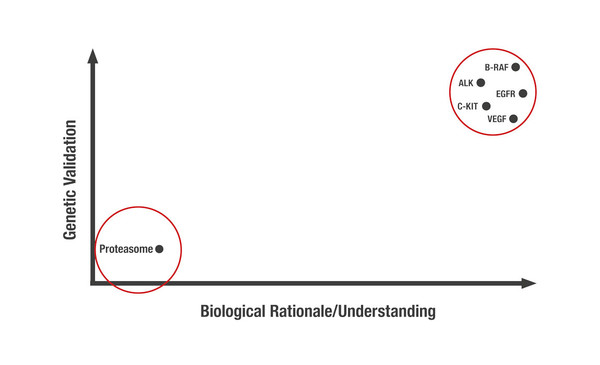
Unsurprisingly, the most successful druggable targets in precision medicine are those that are both genetically validated – either in vitro or in vivo – and are supported by in-depth biological understanding (Figure 1).
However, some targets meeting both features remain undruggable, despite extensive evaluation. So, which strategies can be employed to ‘drug the undruggable’? One way is to exploit epistatic relationships by targeting druggable critical downstream effectors. An example of this strategy is the use of vascular endothelial growth factor (VEGF) inhibitors in the treatment of renal cancers. Overexpression of VEGF occurs after inactivation of von Hippel Lindau (pVHL) protein, a common initiating event in renal cancers, and subsequent dysregulation of hypoxia-inducible factor (HIF) transcription factors, an alteration that was previously considered undruggable. We are also rediscovering the importance of allosteric inhibitors or degraders – molecules that bind to the allosteric site of an enzyme to elicit protein-fold changes – as a means to indirectly inactivate proteins. More recently, the concept of molecular glues leading to targeted protein degradation or sequestration has come to the forefront of research.
It is my belief that despite advances in precision medicine, long-term cancer control requires combination therapy. The first principle of combination therapy is to combine agents with distinct mechanisms of action to limit the probability of cross-resistance occurring. Although it is tempting to use combinations of medicines with interdependent mechanisms of action to achieve synergistic effects, this has to be balanced with the increased risk of cross-resistance and potentially greater toxicity. From my perspective, empirical combinations that include relatively specific and potent molecules with distinct targets and mechanisms of action may prove superior to more elegant combinations of targeted agents with interdependent actions. We also need to move away from the mindset of treating cancer with monotherapies, especially when those monotherapies carry off-target activities. Taking VEGF inhibitors in renal cancer as an example, some of the most effective agents are not highly specific for VEGF, and although their action against other targets may contribute to their efficacy as monotherapy, it has made their use in combination therapy less compelling owing to increased toxicity.
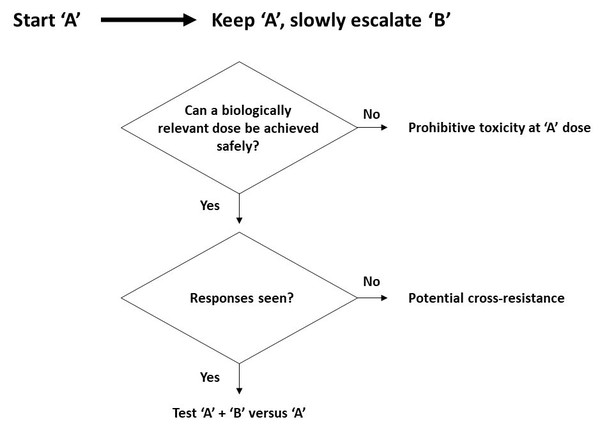
Further, a paradigm shift is needed in the evaluation of drug combinations. The traditional approach implies switching patients to a different treatment at the time of disease progression, while a new paradigm should be built on adding in and slowly escalating a new medicine while continuing treatment with the initial agent (Figure 2).
a)
b)
With the new paradigm in place, one can more quickly address whether two drugs can be safely combined and whether they are likely to be cross-resistant with one another, thus leading the way to more efficient formal testing of new potential combinations in a broader group of patients.
Kaelin W. (Re)emerging Principles of Cancer Care. ESMO Congress 2021
Keynote Lecture – (Re)emerging Principles of Cancer Care, 18.09.2021, h. 12:45 – 13:15, Channel 1