Initial delays to antibody production are followed by similar levels of vaccine efficacy after a second vaccine dose
The latest findings from five studies presented at the ESMO Congress 2021 help cut short the debate on the safety and efficacy of COVID vaccines in cancer patients and re-affirm ESMO’s recommendations for early vaccination of this high-priority population, as outlined in the ESMO ten statements, which were updated in April 2021. Patients with cancer or a history of cancer were under-represented in the initial pivotal vaccine trials, despite mortality due to COVID-19 is higher in this group compared with the general population.
Cancer patients seem to respond better to the vaccination after the second dose and if they had a previous diagnosis of COVID-19
“Overall, these results suggest that cancer patients respond to the vaccination with a good safety profile, and they seem to respond better after the second dose and if they had a previous diagnosis of COVID-19, thus supporting the concept of the booster in this population,” explains Dr Marina Chiara Garassino from the University of Chicago, IL, USA, commenting on the studies’ findings. “Haematological malignancies and patients on chemotherapy could have less seroconversion than others, so it is important to continue to collect these data to see possible differences across tumour types and treatments.”
COVID vaccines appear to have high efficacy in patients with cancer. Results presented today from a post-hoc subgroup analysis of 3,813 patients in the phase III BNT162b2 vaccine trial who had a history of past or active malignancy or neoplasm (including benign neoplasms) revealed that vaccine efficacy was 94.4% (95% CI 85.2–98.5) in patients with a malignancy or neoplasm and 92.9% (95% CI 77.6–98.6) in those with malignancy only (Abstract 1558O). This was similar to the vaccine efficacy of 91.1% (95% CI 88.8–93.0) in the overall trial population, comprising 43,331 evaluable participants aged ≥12 years. Safety findings were similar for patients with cancer and those of the overall trial population, both in incidence and type of common adverse events (N Engl J Med 2020;383:2603-2615). No vaccine-related deaths were reported.
A delayed antibody response to vaccination - which was enhanced by prior exposure to the SARS-CoV-2 virus in patients with cancer - was reported in some studies. The first results from the non-randomised, prospective, phase IV CoVigi study in patients receiving anticancer therapy showed that peak of anti-S antibody responses following vaccination (≥98% of patients received the Pfizer-BioNTech COVID-19 vaccine) occurred approximately 2 months after a first vaccine dose; although an impaired antibody response was found in some patients, after 3 months all vaccinated patients had detectable anti-S antibody levels (Abstract 1563MO). There was an enhanced anti-S antibody response after the first vaccine dose in both cancer patients and healthy volunteers among those who had recovered from prior infection with the virus. After a second vaccine dose, levels of anti-S antibodies in cancer patients who had prior COVID-19 infection were comparable to levels in healthy volunteers, whereas levels were significantly lower in COVID-19-naïve patients.
Similarly, in another prospective study, there was delayed antibody production in 232 patients with solid tumours on active treatment with chemotherapy, immunotherapy or biological therapy compared with non-cancer controls (Abstract 1559O). After the first dose of the Pfizer-BioNTech COVID-19 vaccine, seroconversion occurred in 29% of cancer patients (84% in age-matched controls), rising to 86% following a second dose. Chemotherapy was associated with a reduction in immunogenicity (odds ratio 0.41; 95% confidence interval [CI] 0.17–0.98). A delayed or insufficient antibody response was also described in another study in 81 patients undergoing treatment for advanced genitourinary cancers, with negative antibody titres reported in 6 of 81 patients (7.4%) receiving immune checkpoint inhibitors (ICI) and 12 of 81 patients (14.8%) receiving non-ICI therapy (Abstract 1564MO). These results were recorded at a median 13.5 and 18 days, respectively, after the first vaccine dose, but 4 patients continued to have negative titres 2 months after vaccination.
Garassino thinks these results have key implications for vaccine scheduling in patients with cancer. “It is important that cancer patients receive at least the second vaccine dose – if not the booster – to offset the lag in seroconversion. Further research must also highlight any differences in seroconversion by tumour type and the duration of protection offered by the vaccines.”
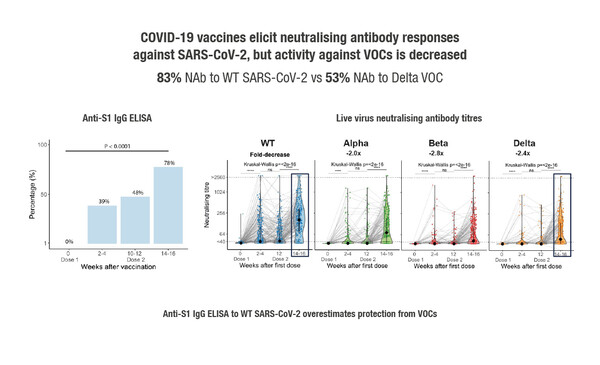
Interim results of the ongoing prospective, longitudinal CAPTURE study, which was designed to characterise functional immune responses to native infection and COVID-19 vaccination in cancer patients, were reported at yesterday’s Presidential Symposium 3 and showed that presence and activity of neutralising antibodies was dependent on cancer type and virus variant (Abstract 1557O). In the infection cohort of 118 patients with confirmed COVID-19 infection – defined as a positive RT-PCR and/or S1 reactive antibodies, there were durable neutralising antibody responses, but these were reduced in patients with haematological malignancies and also against variants of concern (VOC). Neutralising responses were unaffected by comorbidities, age, gender or COVID-19 severity. In general, anticancer treatment did not impact humoral (neutralising antibodies) or cellular (CD4+ and CD8+ T-cell activation) responses to COVID-19 infection, although humoral responses were reduced by anti-CD20 therapy, and cellular responses by ICI. In the vaccine cohort of 585 patients who had received the AstraZeneca (74%), Pfizer-BioNTech (26%) or other (<0.5%) vaccine type, 78% of infection-naïve patients had seroconversion (detection of anti-S IgG antibodies) after their second dose, but activity against VOC was decreased (Figure). Neutralising activity – particularly against VOC – was significantly reduced in patients with haematological malignancies but was unaffected by cancer treatment.
“Evidence collected so far suggests that vaccination protects a large number of patients after the second dose, although protection may be reduced against some VOC,” says Garassino. “Currently, results are unclear regarding the level of protection offered by vaccines in haematological malignancies and patients on chemotherapy. However, of note, the safety profile is favourable. Education on vaccination in patients and carers must continue, alongside self-protection measures.”
Obermannova R et al. CoVigi phase IV multicentric trial evaluating COVID-19 vaccination adverse events and immune response dynamics in cancer patients: First results on antibody and cellular immunity. ESMO Congress 2021, 1563MO
Mini oral session – SARS-CoV-2 and cancer, 21.09.2021, h. 16:25 - 16:30, Channel 2
Waldhorn I et al. Efficacy and toxicity of BNT162b2 vaccine in cancer patients. ESMO Congress 2021, 1559O
Proffered Paper session – SARS-CoV-2 and cancer, 21.09.2021, h. 13:40 – 13:50, Channel 5
Salgia S K et al. Characterization of COVID-19 vaccination response by antibody (Ab) titer and T-cell receptor (TCR) sequencing in patients (pts) with advanced genitourinary (GU) cancers. ESMO Congress 2021, 1564MO
Mini oral session – SARS-CoV-2 and cancer, 21.09.2021, h. 16:30 – 16:35, Channel 2
Thomas S J et al. COVID-19 vaccine in participants (ptcpts) with cancer: Subgroup analysis of efficacy/safety from a global phase III randomized trial of the BNT162b2 (tozinameran) mRNA vaccine. ESMO Congress 2021, 1558O
Proffered Paper session – SARS-CoV-2 and cancer, 21.9.2021, h. 13:30 – 14:50, Channel 5
Shepherd S T et al. Adaptive immunity toSARS-CoV-2 infection and vaccination in cancer patients: The CAPTURE study. ESMO Congress 2021, 1557O
Presidential symposium 3, 20.9.2021, h. 15:30 – 15:45, Channel 1