Most, but not all, patients on chemotherapy or immunotherapy for solid tumours had an adequate immune response after two doses of mRNA-1273 vaccination, with no new safety concerns
Results presented today at Presidential Symposium 3 give an insight into the impact of chemotherapy and immunotherapy on the immunogenicity and safety of vaccination against SARS-CoV-2. A prospective trial compared immune response and safety in four cohorts: individuals without cancer, and patients with solid tumours treated with immunotherapy, chemotherapy or chemo-immunotherapy (LBA8). In total, 743 of 791 enrolled participants were antibody-negative at baseline, received two mRNA-1273 vaccinations 28 days apart and completed assessments on Day 28 after the second vaccination (per protocol population).
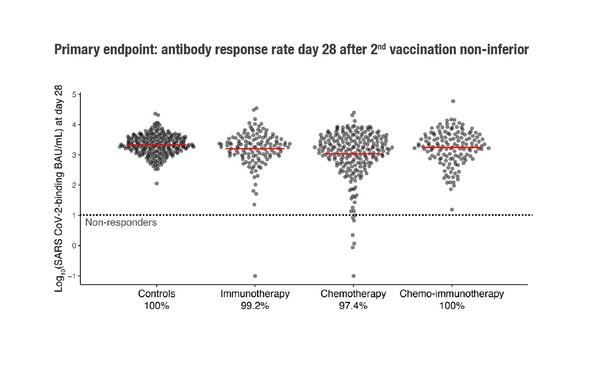
The primary endpoint of SARS-CoV-2 Spike S1-specific IgG serum antibody response (>10 binding antibody units (BAU)/mL) 28 days after the second vaccination was achieved by 97.4–100% of participants, with a non-inferior response between patients with cancer and individuals without cancer.
After the first vaccination, an adequate response – defined as a cut-off level of 300 BAU/mL based on the neutralising capacity of vaccine-induced antibodies – was achieved by 66.0% of individuals without cancer, 37.1% on immunotherapy, 32.5% on chemotherapy and 33.3% on chemo-immunotherapy. In the 28 days after the second vaccination, the proportion with an adequate response increased to 99.6% of individuals without cancer, 93.1% on immunotherapy, 83.8% on chemotherapy and 88.8% on chemo-immunotherapy. Spike-specific T-cell responses were detected in 46.8% of inadequate and non-responders.
The authors concluded that as a substantial minority – up to approximately 16% – lacked an adequate response, an additional booster may be needed in these patients. No new safety signals were observed.
In response to questions posed in the Q&A session on optimal time to give the vaccine in patients receiving cancer therapy, Dr Sjoukje Oosting, the study presenter from University Medical Center Groningen, The Netherlands, replied, “We did not take into account the day of chemotherapy in the vaccine schedule, as we had a very short period of access to the vaccine. We performed an exploratory analysis to look at responses based on time between chemotherapy and vaccination, and found no clear signal of a difference in response, which is in line with previous studies of the flu vaccine in patients receiving chemotherapy that showed no difference between patients receiving the vaccine on Day 1 or Day 11”.
“The most important messages we can take from these prospective data are that vaccination against SARS-CoV-2 appears to be safe in patients with cancer, and that patients with solid tumours mount a robust response comparable to that of healthy controls, that is independent of the therapy they receive,” comments Prof. Marie von Lilienfeld-Toal from University Hospital Jena, Germany. “It will be interesting to learn more about the clinical efficacy with longer follow-up, including how this correlates with immunological outcome parameters; however, the clear message seems to be to vaccinate patients with cancer,” she concludes.
Oosting S et al. Vaccination against SARS-CoV-2 in patients receiving chemotherapy, immunotherapy, or chemo-immunotherapy for solid tumors. ESMO Congress 2021, Abstract LBA8
Presidential symposium 3, 20.09.2021, h. 15:45 – 16:00, Channel 1