Studies underline that a tumour-agnostic approach may help improve treatment options
“Changing how we name all cancers – shifting from the current primary site approach to a more molecular classification – may help remove some of the obstacles to accessible targeted treatments for patients with cancer of unknown primary (CUP). Although this is a long way off, it should be an aspirational goal for the whole oncology community,” states Prof. Alwin Krämer from the German Cancer Research Center (DKFZ) and the University of Heidelberg, Germany. Research into CUP reflects the complexity of targeting cancer beyond its origin or location. While its incidence is decreasing slightly, CUP remains a clinical dilemma associated with a high mortality rate (Ann Oncol. 2023;34:228–246).
Studies presented at the ESMO Asia Congress 2025 (Singapore, 5–7 December) highlight the need to perform routine comprehensive genomic profiling, preferably with whole-genome and transcriptome sequencing (WGTS), and then provide access to the respective targeted therapy to match any identified molecular alterations to improve survival.
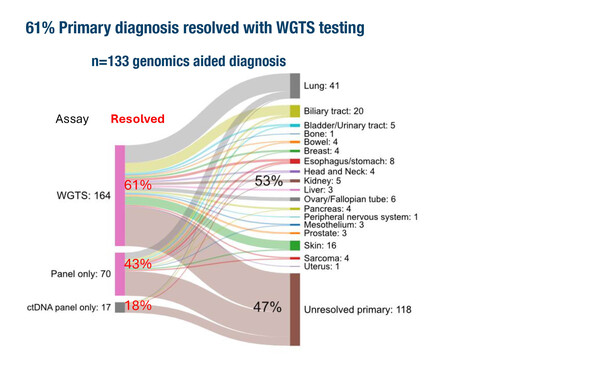
In an Australian prospective observational study, WGTS was more effective than comprehensive panel testing and enabled tissue-of-origin diagnosis in more than half of CUP cases (Abstract 762MO, key results in the table below). Among 251 patients with clinicopathology-unresolved CUP who underwent at least one genomic test, a likely tissue-of-origin diagnosis was reported for 61% of patients with WGTS testing versus 43% with tissue panel-only testing.
Clinicians reported that genomic results had some impact on treatment planning for 165 patients (66%). Of note, 38 patients (15%) received a change of treatment, which was to the standard of care for that tissue-of-origin in 31 patients (82%) and to targeted therapy in 13 patients (34%). An improvement in median WGTS turnaround time was observed from 42 days in the first year of the study to 27 days in the second year.
“This presentation confirms the authors’ previous smaller study demonstrating that WGTS improves tissue-of-origin identification over panel testing (Nat Commun. 2025;16:4422),” notes Krämer. “Here the authors also concluded that the impact on testing was mainly through resolving the tissue-of-origin rather than using agnostic molecularly guided treatments. This likely reflects the Australian healthcare system where a treatment can only be assigned if the primary site is known. More generally, what is most important is that the patient has access to and receives the right targeted treatment.” The CUPISCO trial has shown previously that a targeted approach to unfavourable CUP – providing molecularly guided therapy – results in longer progression-free survival than standard platinum-based chemotherapy (Lancet. 2024;404:527–539).
The impact of assigning a primary site was analysed in a 10-year retrospective study from the Netherlands (Abstract 763MO, key results in the table below). Of 512 patients studied with CUP, 257 were later assigned a primary site, while 255 remained unclassified. A likely primary tumour site was more often assigned in patients with more diagnostic hints; however, more diagnostic clues often corresponded to a more advanced disease state and poorer baseline prognosis. Median survival was 531 days in patients who later had an assigned primary site and 355 days in those with unclassified CUP (p=0.326). The assigned versus the unclassified group were more likely to receive chemotherapy (43.2% versus 33.7%; p=0.03), hormonal therapy (16.0% versus 5.5%; p=0.0002), immunotherapy (12.1% versus 3.9%; p=0.001), targeted therapy (14.4% versus 5.1%; p=0.0007) and multimodal treatments (47.9% versus 26.7%; p=0.0001).
“Despite their greater tumour burden, outcomes were no worse in the assigned group and this is likely because of better access to innovative treatments, such as targeted and immunotherapy. Again, this presentation highlights that access to targeted therapy is key and currently easier if you can give the disease a name,” observes Krämer. Although targeted therapies and immunotherapies are now standard for many tumour types, they are not yet accessible for most patients with CUP, and “this should be an important focus to improve prognosis moving forward,” concludes Krämer.
At a glance:
Rebello RJ, et al. Clinical whole genome and transcriptome sequencing for cancer of unknown primary: An Australian prospective observational study. ESMO Asia Congress 2025 - Abstract 762MO
- CUP and molecular tests reported (N=251)
- 61% had a likely TOO diagnosis with WGTS vs 43% with panel-only testing
- 15% received a change of treatment informed by genomic testing
Rostami S, et al. Multimodal profiling of cancer of unknown primary with and without subsequent diagnosis: Does assigning a primary tumor matter? ESMO Asia Congress 2025 - Abstract 763MO
- CUP (N=512)
- Later assigned a primary site: n=257; remained unclassified: n=255
- Median survival: 531 days in assigned primary site vs 355 days in unclassified (p=0.326)